Live attenuated tetravalent dengue virus host range vaccine is immunogenic in African green monkeys following a single vaccination
- PMID: 24696467
- PMCID: PMC4054385
- DOI: 10.1128/JVI.00541-14
Live attenuated tetravalent dengue virus host range vaccine is immunogenic in African green monkeys following a single vaccination
Abstract
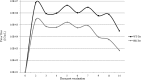
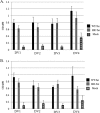
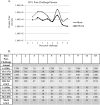
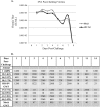
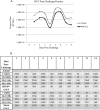
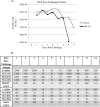
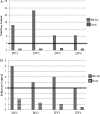
The causative agent of dengue fever, dengue virus (DENV), is transmitted by mosquitoes, and as distribution of these insects has expanded, so has dengue-related disease. DENV is a member of the Flaviviridae family and has 4 distinct serotypes (DENV-1, -2, -3, and -4). No lasting cross protection is afforded to heterologous serotypes following infection by any one of the individual serotypes. The presence of nonneutralizing antibodies to one serotype can facilitate the occurrence of more-severe dengue hemorrhagic fever through immune enhancement upon infection with a second serotype. For this reason, the development of a safe, tetravalent vaccine to produce a balanced immune response to all four serotypes is critical. We have developed a novel approach to produce safe and effective live-attenuated vaccines for DENV and other insect-borne viruses. Host range (HR) mutants of each DENV serotype were created by truncating transmembrane domain 1 of the E protein and selecting for strains of DENV that replicated well in insect cells but not mammalian cells. These vaccine strains were tested for immunogenicity in African green monkeys (AGMs). No vaccine-related adverse events occurred. The vaccine strains were confirmed to be attenuated in vivo by infectious center assay (ICA). Analysis by 50% plaque reduction neutralization test (PRNT50) established that by day 62 postvaccination, 100% of animals seroconverted to DENV-1, -2, -3, and -4. Additionally, the DENV HR tetravalent vaccine (HR-Tet) showed a tetravalent anamnestic immune response in 100% (16/16) of AGMs after challenge with wild-type (WT) DENV strains.
Importance: We have generated a live attenuated viral (LAV) vaccine capable of eliciting a strong immune response in African green monkeys (AGMs) in a single dose. This vaccine is delivered by injecting one of four attenuated serotypes into each limb of the animal. 100% of animals given the vaccine generated antibodies against all 4 serotypes, and this response was found to be balanced in nature. This is also one of the first studies of dengue in AGMs, and our study suggests that viremia and antibody response in AGMs may be similar to those seen in DENV infection in humans.
Copyright © 2014, American Society for Microbiology. All Rights Reserved.
Figures

References
-
- WHO. 2014. Dengue and severe dengue. Fact sheet no. 17. WHO, Geneva, Switzerland: http://www.who.int/mediacentre/factsheets/fs117/en/
-
- Halstead SB. 1980. Immunological parameters of Togavirus disease syndromes, p 107–168 In Schlesinger RW. (ed), The togaviruses: biology, structure, replication. Academic Press, New York, NY
-
- WHO. 2012. Dengue/dengue haemorrhagic fever. WHO, Geneva, Switzerland: http://www.who.int/csr/disease/dengue/en/
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

