Characterizing functional domains for TIM-mediated enveloped virus entry
- PMID: 24696470
- PMCID: PMC4054341
- DOI: 10.1128/JVI.00300-14
Characterizing functional domains for TIM-mediated enveloped virus entry
Abstract
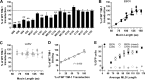
T-cell immunoglobulin and mucin domain 1 (TIM-1) and other TIM family members were recently identified as phosphatidylserine (PtdSer)-mediated virus entry-enhancing receptors (PVEERs). These proteins enhance entry of Ebola virus (EBOV) and other viruses by binding PtdSer on the viral envelope, concentrating virus on the cell surface, and promoting subsequent internalization. The PtdSer-binding activity of the immunoglobulin-like variable (IgV) domain is essential for both virus binding and internalization by TIM-1. However, TIM-3, whose IgV domain also binds PtdSer, does not effectively enhance virus entry, indicating that other domains of TIM proteins are functionally important. Here, we investigate the domains supporting enhancement of enveloped virus entry, thereby defining the features necessary for a functional PVEER. Using a variety of chimeras and deletion mutants, we found that in addition to a functional PtdSer-binding domain PVEERs require a stalk domain of sufficient length, containing sequences that promote an extended structure. Neither the cytoplasmic nor the transmembrane domain of TIM-1 is essential for enhancing virus entry, provided the protein is still plasma membrane bound. Based on these defined characteristics, we generated a mimic lacking TIM sequences and composed of annexin V, the mucin-like domain of α-dystroglycan, and a glycophosphatidylinositol anchor that functioned as a PVEER to enhance transduction of virions displaying Ebola, Chikungunya, Ross River, or Sindbis virus glycoproteins. This identification of the key features necessary for PtdSer-mediated enhancement of virus entry provides a basis for more effective recognition of unknown PVEERs.
Importance: T-cell immunoglobulin and mucin domain 1 (TIM-1) and other TIM family members are recently identified phosphatidylserine (PtdSer)-mediated virus entry-enhancing receptors (PVEERs). These proteins enhance virus entry by binding the phospholipid, PtdSer, present on the viral membrane. While it is known that the PtdSer binding is essential for the PVEER function of TIM-1, TIM-3 shares this binding activity but does not enhance virus entry. No comprehensive studies have been done to characterize the other domains of TIM-1. In this study, using a variety of chimeric proteins and deletion mutants, we define the features necessary for a functional PVEER. With these features in mind, we generated a TIM-1 mimic using functionally similar domains from other proteins. This mimic, like TIM-1, effectively enhanced transduction. These studies provide insight into the key features necessary for PVEERs and will allow for more effective identification of unknown PVEERs.
Copyright © 2014, American Society for Microbiology. All Rights Reserved.
Figures



References
-
- Jemielity S, Wang JJ, Chan YK, Ahmed AA, Li W, Monahan S, Bu X, Farzan M, Freeman GJ, Umetsu DT, Dekruyff RH, Choe H. 2013. TIM-family proteins promote infection of multiple enveloped viruses through virion-associated phosphatidylserine. PLoS Pathog. 9:e1003232. 10.1371/journal.ppat.1003232 - DOI - PMC - PubMed
-
- Kondratowicz AS, Lennemann NJ, Sinn PL, Davey RA, Hunt CL, Moller-Tank S, Meyerholz DK, Rennert P, Mullins RF, Brindley M, Sandersfeld LM, Quinn K, Weller M, McCray PB, Jr, Chiorini J, Maury W. 2011. T-cell immunoglobulin and mucin domain 1 (TIM-1) is a receptor for Zaire Ebolavirus and Lake Victoria Marburgvirus. Proc. Natl. Acad. Sci. U. S. A. 108:8426–8431. 10.1073/pnas.1019030108 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

