Phylogenetic distribution and evolution of the linked RNA-binding and NOT1-binding domains in the tristetraprolin family of tandem CCCH zinc finger proteins
- PMID: 24697206
- PMCID: PMC3976581
- DOI: 10.1089/jir.2013.0150
Phylogenetic distribution and evolution of the linked RNA-binding and NOT1-binding domains in the tristetraprolin family of tandem CCCH zinc finger proteins
Abstract
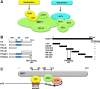
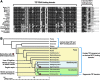
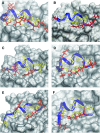
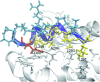
In humans, the tristetraprolin or TTP family of CCCH tandem zinc finger (TZF) proteins comprises 3 members, encoded by the genes ZFP36, ZFP36L1, and ZFP36L2. These proteins have direct orthologues in essentially all vertebrates studied, with the exception of birds, which appear to lack a version of ZFP36. Additional family members are found in rodents, amphibians, and fish. In general, the encoded proteins contain 2 critical macromolecular interaction domains: the CCCH TZF domain, which is necessary for high-affinity binding to AU-rich elements in mRNA; and an extreme C-terminal domain that, in the case of TTP, interacts with NOT1, the scaffold of a large multi-protein complex that contains deadenylases. TTP and its related proteins act by first binding to AU-rich elements in mRNA, and then recruiting deadenylases to the mRNA, where they can processively remove the adenosine residues from the poly(A) tail. Highly conserved TZF domains have been found in unicellular eukaryotes such as yeasts, and these domains can bind AU-rich elements that resemble those bound by the mammalian proteins. However, certain fungi appear to lack proteins with intact TZF domains, and the TTP family proteins that are expressed in other fungi often lack the characteristic C-terminal NOT1 binding domain found in the mammalian proteins. For these reasons, we investigated the phylogenetic distribution of the relevant sequences in available databases. Both domains are present in family member proteins from most lineages of eukaryotes, suggesting their mutual presence in a common ancestor. However, the vertebrate type of NOT1-binding domain is missing in most fungi, and the TZF domain itself has disappeared or degenerated in recently evolved fungi. Nonetheless, both domains are present together in the proteins from several unicellular eukaryotes, including at least 1 fungus, and they seem to have remained together during the evolution of metazoans.
Figures

References
-
- Belloc E, Mendez R. 2008. A deadenylation negative feedback mechanism governs meiotic metaphase arrest. Nature 452:1017–1021 - PubMed
-
- Beutler B, Moresco EM. 2008. The forward genetic dissection of afferent innate immunity. Curr Top Microbiol Immunol 321:3–26 - PubMed
-
- Blackshear PJ, Phillips RS, Ghosh S, Ramos SB, Richfield EK, Lai WS. 2005. Zfp36l3, a rodent X chromosome gene encoding a placenta-specific member of the Tristetraprolin family of CCCH tandem zinc finger proteins. Biol Reprod 73:297–307 - PubMed
-
- Carballo E, Gilkeson GS, Blackshear PJ. 1997. Bone marrow transplantation reproduces the tristetraprolin-deficiency syndrome in recombination activating gene-2 (−/−) mice. Evidence that monocyte/macrophage progenitors may be responsible for TNFalpha overproduction. J Clin Invest 100:986–995 - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

