The bruton tyrosine kinase inhibitor ibrutinib (PCI-32765) blocks hairy cell leukaemia survival, proliferation and B cell receptor signalling: a new therapeutic approach
- PMID: 24697238
- PMCID: PMC4104473
- DOI: 10.1111/bjh.12867
The bruton tyrosine kinase inhibitor ibrutinib (PCI-32765) blocks hairy cell leukaemia survival, proliferation and B cell receptor signalling: a new therapeutic approach
Abstract
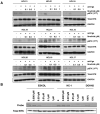
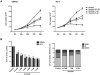
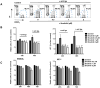
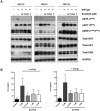
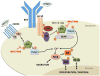
B cell receptor (BCR) signalling plays a critical role in the progression of several B-cell malignancies, but its role in hairy cell leukaemia (HCL) is ambiguous. Bruton tyrosine kinase (BTK), a key player in BCR signalling, as well as B cell migration and adhesion, can be targeted with ibrutinib, a selective, irreversible BTK inhibitor. We analysed BTK expression and function in HCL and analysed the effects of ibrutinib on HCL cells. We demonstrated uniform BTK protein expression in HCL cells. Ibrutinib significantly inhibited HCL proliferation and cell cycle progression. Accordingly, ibrutinib also reduced HCL cell survival after BCR triggering with anti-immunoglobulins and abrogated the activation of kinases downstream of the BCR (PI3K and MAPK). Ibrutinib also inhibited BCR-dependent secretion of the chemokines CCL3 and CCL4 by HCL cells. Interestingly, ibrutinib inhibited also CXCL12-induced signalling, a key pathway for bone marrow homing. Collectively, our data support the clinical development of ibrutinib in patients with HCL.
Keywords: B cell receptor; bruton tyrosine kinase; hairy cell leukaemia; ibrutinib; microenvironment.
© 2014 John Wiley & Sons Ltd.
Conflict of interest statement
Figures

References
-
- Advani RH, Buggy JJ, Sharman JP, Smith SM, Boyd TE, Grant B, Kolibaba KS, Furman RR, Rodriguez S, Chang BY, Sukbuntherng J, Izumi R, Hamdy A, Hedrick E, Fowler NH. Bruton tyrosine kinase inhibitor ibrutinib (PCI-32765) has significant activity in patients with relapsed/refractory B-cell malignancies. J Clin Oncol. 2013;31:88–94. - PMC - PubMed
-
- Burger JA, Buggy JJ. Emerging drug profiles: Bruton tyrosine kinase (BTK) inhibitor ibrutinib (PCI-32765) Leuk Lymphoma 2013 - PubMed
-
- Burger JA, Kipps TJ. CXCR4: a key receptor in the crosstalk between tumor cells and their microenvironment. BLOOD. 2006;107:1761–1767. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

