Does PKC activation increase the homologous desensitization of μ opioid receptors?
- PMID: 24697621
- PMCID: PMC4292970
- DOI: 10.1111/bph.12712
Does PKC activation increase the homologous desensitization of μ opioid receptors?
Abstract
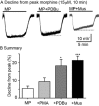
Background and purpose: This study examined the role of agents known to activate PKC on morphine-induced desensitization of μ-opioid receptors (MOP receptors) in brain slices containing locus coeruleus neurons.
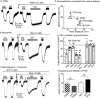
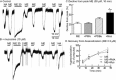
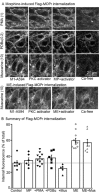
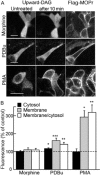
Experimental approach: Intracellular recordings were obtained from rat locus coeruleus neurons. Two measurements were used to characterize desensitization, the decline in hyperpolarization induced by application of a saturating concentration of agonist (acute desensitization) and the decrease in hyperpolarization induced by a subsaturating concentration of [Met](5) enkephalin (ME) following washout of the saturating concentration (sustained desensitization). Internalization of MOP receptors was studied in brain slices prepared from transgenic mice expressing Flag-MOP receptors. The subcellular distribution of activated PKC was examined using a novel fluorescent sensor of PKC in HEK293 cells.
Key results: The phorbol esters (PMA and PDBu) and muscarine increased acute desensitization induced by a saturating concentration of morphine and ME. These effects were not sensitive to staurosporine. Staurosporine did not block the decline in hyperpolarization induced by muscarine. PDBu and muscarine did not affect sustained desensitization induced by ME nor did phorbol esters or muscarine change the trafficking of MOP receptors induced by morphine or ME. The distribution of activated PKC measured in HEK293 cells differed depending on which phorbol ester was applied.
Conclusions and implications: This study demonstrates a distinct difference in two measurements that are often used to evaluate desensitization. The measure of decline correlated well with the reduction in peak amplitudes caused by PKC activators implicating the modification of other factors rather than MOP receptors.
Linked articles: This article is part of a themed section on Opioids: New Pathways to Functional Selectivity. To view the other articles in this section visit http://dx.doi.org/10.1111/bph.2015.172.issue-2.
Keywords: PKC; desensitization; fluorescent PKC sensor; hyperpolarization; internalization; muscarine; phorbol esters; staurosporine; μ opioid receptor.
© 2014 The British Pharmacological Society.
Figures
References
-
- Bailey CP, Kelly E, Henderson G. Protein kinase C activation enhances morphine-induced rapid desensitization of mu-opioid receptors in mature rat locus coeruleus neurons. Mol Pharmacol. 2004;66:1592–1598. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

