Safety of switching from vitamin K antagonists to dabigatran or rivaroxaban in daily care--results from the Dresden NOAC registry
- PMID: 24697922
- PMCID: PMC4239984
- DOI: 10.1111/bcp.12391
Safety of switching from vitamin K antagonists to dabigatran or rivaroxaban in daily care--results from the Dresden NOAC registry
Abstract
Aim: Vitamin-K antagonists (VKA) and non-vitamin-K dependent oral anticoagulants (NOAC) have been approved for anticoagulation in venous thromboembolism (VTE) and atrial fibrillation and patients previously treated with VKA are switched to NOAC therapy. Safety data for this switching are urgently needed.
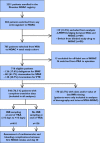
Methods: Using data from a large regional prospective registry of daily care NOAC patients, we evaluated the safety of switching anticoagulation from VKA to dabigatran or rivaroxaban. Switching procedures and cardiovascular and bleeding events occurring within 30 days after switching were centrally adjudicated.
Results: Between 1 October 2011 and 18 June 2013, 2231 patients were enrolled. Of these, 716 patients were switched from VKA to NOAC. Only 410 of the 546 evaluable patients (75.1%) had a recorded INR measurement within the 10 days preceding or following the end of VKA treatment (mean INR 2.4). As of day 30, major bleeding complications were rare (0.3%; 95% CI 0.0, 1.0) with an overall bleeding rate of 12.2% (95% CI 9.8, 14.8). Major cardiovascular events occurred in 0.8% (95% CI 0.3, 1.8). There was no significant difference in outcome event rates between the subgroups of patients with or without INR testing.
Conclusion: In daily care, only 75% of VKA patients have an INR measurement documented before NOAC are started. On average, NOAC are started within 2 to 5 days after the last intake of VKA. However, at 30 days follow-up cardiovascular events or major bleedings were rare both in patients with and without INR testing. However, switching procedures need to be further evaluated in larger cohorts of patients.
Keywords: VKA; bleeding risk; novel oral anticoagulants; switching.
© 2014 The British Pharmacological Society.
Figures
Similar articles
-
Rivaroxaban and dabigatran in patients undergoing catheter ablation of atrial fibrillation.Europace. 2014 Aug;16(8):1137-44. doi: 10.1093/europace/euu007. Epub 2014 Feb 18. Europace. 2014. PMID: 24550347
-
Non-vitamin K antagonist oral anticoagulants in cardiovascular disease management: evidence and unanswered questions.J Clin Pharm Ther. 2014 Apr;39(2):118-35. doi: 10.1111/jcpt.12122. Epub 2014 Jan 3. J Clin Pharm Ther. 2014. PMID: 24383983 Review.
-
[Comparison of the safety of rivaroxaban versus dabigatran therapy in patients with persistent atrial fibrillation].Pol Merkur Lekarski. 2014 Nov;37(221):261-4. Pol Merkur Lekarski. 2014. PMID: 25546985 Clinical Trial. Polish.
-
Changes in Oral Anticoagulation Therapy over One Year in 51,000 Atrial Fibrillation Patients at Risk for Stroke: A Practice-Derived Study.Thromb Haemost. 2019 Jun;119(6):882-893. doi: 10.1055/s-0039-1683428. Epub 2019 Mar 21. Thromb Haemost. 2019. PMID: 30900220
-
Bleeding with dabigatran, rivaroxaban, apixaban. No antidote, and little clinical experience.Prescrire Int. 2013 Jun;22(139):155-9. Prescrire Int. 2013. PMID: 23866358 Review.
Cited by
-
New oral anti-coagulants versus vitamin K antagonists in high thromboembolic risk patients.PLoS One. 2019 Oct 7;14(10):e0222762. doi: 10.1371/journal.pone.0222762. eCollection 2019. PLoS One. 2019. PMID: 31589620 Free PMC article.
-
Strengths and weaknesses of 'real-world' studies involving non-vitamin K antagonist oral anticoagulants.Open Heart. 2018 Apr 21;5(1):e000788. doi: 10.1136/openhrt-2018-000788. eCollection 2018. Open Heart. 2018. PMID: 29713485 Free PMC article. Review.
-
Novel Anticoagulant Treatment for Pulmonary Embolism with Direct Oral Anticoagulants Phase 3 Trials and Clinical Practice.Semin Intervent Radiol. 2018 Jun;35(2):83-91. doi: 10.1055/s-0038-1642622. Epub 2018 Jun 4. Semin Intervent Radiol. 2018. PMID: 29872242 Free PMC article. Review.
-
Left Atrial Appendage Thrombus Resolution with Reduced Dose Apixaban.J Atr Fibrillation. 2015 Jun 30;8(1):1182. doi: 10.4022/jafib.1182. eCollection 2015 Jun-Jul. J Atr Fibrillation. 2015. PMID: 27957170 Free PMC article.
-
High number of newly initiated direct oral anticoagulant users switch to alternate anticoagulant therapy.J Thromb Thrombolysis. 2017 Nov;44(4):435-441. doi: 10.1007/s11239-017-1565-2. J Thromb Thrombolysis. 2017. PMID: 29027097
References
-
- Connolly SJ, Ezekowitz MD, Yusuf S, Eikelboom J, Oldgren J, Parekh A, Pogue J, Reilly PA, Themeles E, Varrone J, Wang S, Alings M, Xavier D, Zhu J, Diaz R, Lewis BS, Darius H, Diener HC, Joyner CD, Wallentin L. Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med. 2009;361:1139–1151. - PubMed
-
- Patel MR, Mahaffey KW, Garg J, Pan G, Singer DE, Hacke W, Breithardt G, Halperin JL, Hankey GJ, Piccini JP, Becker RC, Nessel CC, Paolini JF, Berkowitz SD, Fox KA, Califf RM. Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N Engl J Med. 2011;365:883–891. - PubMed
-
- Bauersachs R, Berkowitz SD, Brenner B, Buller HR, Decousus H, Gallus AS, Lensing AW, Misselwitz F, Prins MH, Raskob GE, Segers A, Verhamme P, Wells P, Agnelli G, Bounameaux H, Cohen A, Davidson BL, Piovella F, Schellong S. Oral rivaroxaban for symptomatic venous thromboembolism. N Engl J Med. 2010;363:2499–2510. - PubMed
-
- SmPC rivaroxaban, June 2013. Available at http://wwwmedicinesorguk/emc/medicine/25586/SPC (accessed 22 August 2013)
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

