Analytical reactivity of 13 commercially available rapid influenza diagnostic tests with H3N2v and recently circulating influenza viruses
- PMID: 24698134
- PMCID: PMC4181808
- DOI: 10.1111/irv.12246
Analytical reactivity of 13 commercially available rapid influenza diagnostic tests with H3N2v and recently circulating influenza viruses
Abstract
Objectives: Rapid influenza diagnostic tests (RIDTs) used widely in clinical practice are simple to use and provide results within 15 minutes; however, reported performance is variable, which causes concern when novel or variant viruses emerge. This study's goal was to assess the analytical reactivity of 13 RIDTs with recently circulating seasonal and H3N2v influenza viruses, using three different viral measures.
Design: Virus stocks were characterized by infectious dose (ID50 ) and nucleoprotein (NP) concentration, diluted at half-log dilutions, and tested with each RIDT and real-time RT-PCR.
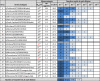
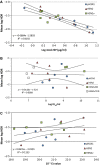
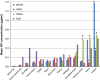
Results: Strong correlation was observed between NP concentration and RIDT reactivity; however, only weak correlation was seen with ID50 or Ct values. Only four RIDTs detected viral NP at the lowest dilution for all influenza A viruses (IAV). Influenza A viruses not detected by more than one RIDT had lower NP levels. Of the 13 RIDTs, 9 had no significant differences in reactivity across IAV when compared to NP levels.
Conclusions: Previous reports of RIDT performance typically compare reactivity based on ID50 titers, which in this study correlated only weakly with proportional amounts of viral NP in prepared virus samples. In the context of the strong correlation of RIDT reactivity with NP concentration, H3N2v was found to be as reactive as seasonal circulating IAV. While these findings may not reflect clinical performance of these RIDTs, measuring NP concentration can be useful in the future to assess comparable reactivity of available RIDTs, or to assess reactivity with newly evolving or emerging viruses.
Keywords: Diagnostic; FDA; H3N2v; influenza; rapid.
© 2014 The Authors. Influenza and Other Respiratory Viruses Published by John Wiley & Sons Ltd.
Figures

Similar articles
-
Analytical detection of influenza A(H3N2)v and other A variant viruses from the USA by rapid influenza diagnostic tests.Influenza Other Respir Viruses. 2013 Jul;7(4):491-6. doi: 10.1111/irv.12017. Epub 2012 Sep 18. Influenza Other Respir Viruses. 2013. PMID: 22984843 Free PMC article.
-
A narrative review of nine commercial point of care influenza tests: an overview of methods, benefits, and drawbacks to rapid influenza diagnostic testing.J Osteopath Med. 2022 Aug 19;123(1):39-47. doi: 10.1515/jom-2022-0065. eCollection 2023 Jan 1. J Osteopath Med. 2022. PMID: 35977624 Review.
-
Comparison of three rapid influenza diagnostic tests with digital readout systems and one conventional rapid influenza diagnostic test.J Clin Lab Anal. 2018 Feb;32(2):e22234. doi: 10.1002/jcla.22234. Epub 2017 Apr 13. J Clin Lab Anal. 2018. PMID: 28407318 Free PMC article.
-
Comparative Evaluation of Three Rapid Influenza Diagnostic Tests for Detection of Influenza A and B Viruses Using RT-PCR as Reference Method.J Med Virol. 2025 Jan;97(1):e70162. doi: 10.1002/jmv.70162. J Med Virol. 2025. PMID: 39812072 Free PMC article.
-
Detection of Influenza A and B Viruses and Respiratory Syncytial Virus by Use of Clinical Laboratory Improvement Amendments of 1988 (CLIA)-Waived Point-of-Care Assays: a Paradigm Shift to Molecular Tests.J Clin Microbiol. 2018 Jun 25;56(7):e00367-18. doi: 10.1128/JCM.00367-18. Print 2018 Jul. J Clin Microbiol. 2018. PMID: 29695519 Free PMC article. Review.
Cited by
-
Analysis of polyclonal and monoclonal antibody to the influenza virus nucleoprotein in different oligomeric states.bioRxiv [Preprint]. 2024 Sep 12:2024.09.12.612748. doi: 10.1101/2024.09.12.612748. bioRxiv. 2024. Update in: Virus Res. 2025 May;355:199563. doi: 10.1016/j.virusres.2025.199563. PMID: 39372734 Free PMC article. Updated. Preprint.
-
Simulated Respiratory Secretion for Use in the Development of Influenza Diagnostic Assays.PLoS One. 2016 Nov 21;11(11):e0166800. doi: 10.1371/journal.pone.0166800. eCollection 2016. PLoS One. 2016. PMID: 27870895 Free PMC article.
-
Analysis of polyclonal and monoclonal antibody to the influenza virus nucleoprotein in different oligomeric states.Virus Res. 2025 May;355:199563. doi: 10.1016/j.virusres.2025.199563. Epub 2025 Mar 24. Virus Res. 2025. PMID: 40139568 Free PMC article.
-
Disposable Autonomous Device for Swab-to-Result Diagnosis of Influenza.Anal Chem. 2017 Jun 6;89(11):5776-5783. doi: 10.1021/acs.analchem.6b04801. Epub 2017 May 8. Anal Chem. 2017. PMID: 28445636 Free PMC article.
-
A panel of anti-influenza virus nucleoprotein antibodies selected from phage-displayed synthetic antibody libraries with rapid diagnostic capability to distinguish diverse influenza virus subtypes.Sci Rep. 2020 Aug 7;10(1):13318. doi: 10.1038/s41598-020-70135-6. Sci Rep. 2020. PMID: 32770098 Free PMC article.
References
-
- Baas C, Barr I, Fouchier R, Kelso A, Hurt A. A comparison of rapid point-of-care tests for the detection of avian influenza A(H7N9) virus, 2013. Euro Surveill. 2013;18:20487. - PubMed
-
- Centers for Disease Control and Prevention. Evaluation of 11 commercially available rapid influenza diagnostic tests–United States, 2011–2012. MMWR Morb Mortal Wkly Rep. 2012;61:873–876. - PubMed
-
- Centers for Disease Control and Prevention. Evaluation of Rapid Influenza Diagnostic Tests for Detection of Novel Influenza A (H1N1) Virus — United States, 2009. MMWR Morb Mortal Wkly Rep. 2009;58:826–829. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

