Analytical reactivity of 13 commercially available rapid influenza diagnostic tests with H3N2v and recently circulating influenza viruses
- PMID: 24698134
- PMCID: PMC4181808
- DOI: 10.1111/irv.12246
Analytical reactivity of 13 commercially available rapid influenza diagnostic tests with H3N2v and recently circulating influenza viruses
Abstract
Objectives: Rapid influenza diagnostic tests (RIDTs) used widely in clinical practice are simple to use and provide results within 15 minutes; however, reported performance is variable, which causes concern when novel or variant viruses emerge. This study's goal was to assess the analytical reactivity of 13 RIDTs with recently circulating seasonal and H3N2v influenza viruses, using three different viral measures.
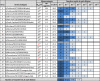
Design: Virus stocks were characterized by infectious dose (ID50 ) and nucleoprotein (NP) concentration, diluted at half-log dilutions, and tested with each RIDT and real-time RT-PCR.
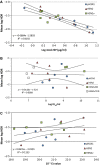
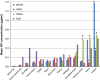
Results: Strong correlation was observed between NP concentration and RIDT reactivity; however, only weak correlation was seen with ID50 or Ct values. Only four RIDTs detected viral NP at the lowest dilution for all influenza A viruses (IAV). Influenza A viruses not detected by more than one RIDT had lower NP levels. Of the 13 RIDTs, 9 had no significant differences in reactivity across IAV when compared to NP levels.
Conclusions: Previous reports of RIDT performance typically compare reactivity based on ID50 titers, which in this study correlated only weakly with proportional amounts of viral NP in prepared virus samples. In the context of the strong correlation of RIDT reactivity with NP concentration, H3N2v was found to be as reactive as seasonal circulating IAV. While these findings may not reflect clinical performance of these RIDTs, measuring NP concentration can be useful in the future to assess comparable reactivity of available RIDTs, or to assess reactivity with newly evolving or emerging viruses.
Keywords: Diagnostic; FDA; H3N2v; influenza; rapid.
© 2014 The Authors. Influenza and Other Respiratory Viruses Published by John Wiley & Sons Ltd.
Figures

References
-
- Baas C, Barr I, Fouchier R, Kelso A, Hurt A. A comparison of rapid point-of-care tests for the detection of avian influenza A(H7N9) virus, 2013. Euro Surveill. 2013;18:20487. - PubMed
-
- Centers for Disease Control and Prevention. Evaluation of 11 commercially available rapid influenza diagnostic tests–United States, 2011–2012. MMWR Morb Mortal Wkly Rep. 2012;61:873–876. - PubMed
-
- Centers for Disease Control and Prevention. Evaluation of Rapid Influenza Diagnostic Tests for Detection of Novel Influenza A (H1N1) Virus — United States, 2009. MMWR Morb Mortal Wkly Rep. 2009;58:826–829. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

