Production and function of pigment epithelium-derived factor in isolated skin keratinocytes
- PMID: 24698153
- PMCID: PMC4151327
- DOI: 10.1111/exd.12411
Production and function of pigment epithelium-derived factor in isolated skin keratinocytes
Abstract
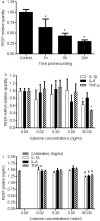
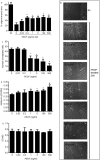
Pigment epithelium-derived factor (PEDF) is a multifunctional factor with potent anti-angiogenic activity that may play a role in skin homoeostasis and wound healing. Analysis of PEDF levels demonstrated that PEDF levels are high in normal skin but quite low in early wounds. As previous studies have suggested that keratinocytes can produce PEDF, we investigated how conditions that mimic those found at sites of injury influence PEDF production by keratinocytes in vitro. Both injury by mechanical disruption (scratch assay) and treatment of human keratinocytes with inflammatory cytokines (IL-1β, IL-6 and TNF-α) inhibited PEDF expression. We next examined how PEDF affects keratinocyte functions that are important in tissue repair. Treatment of keratinocytes with exogenous PEDF enhanced keratinocyte adhesion, therefore impairing migration, while having no effect on cell proliferation. The results suggest that modulation of PEDF levels may play a pivotal role in skin homoeostasis and the response of keratinocytes to injury or inflammatory insults.
Keywords: cytokine; in vitro; keratinocyte; pigment epithelium-derived factor; wound.
© 2014 John Wiley & Sons A/S. Published by John Wiley & Sons Ltd.
Figures
Similar articles
-
Pigment Epithelium-Derived Factor (PEDF) as a Regulator of Wound Angiogenesis.Sci Rep. 2018 Jul 24;8(1):11142. doi: 10.1038/s41598-018-29465-9. Sci Rep. 2018. PMID: 30042381 Free PMC article.
-
Pigment epithelium-derived factor plays an inhibitory role in proliferation and migration of HaCaT cells.Mol Biol Rep. 2011 Mar;38(3):2099-105. doi: 10.1007/s11033-010-0336-3. Epub 2010 Sep 21. Mol Biol Rep. 2011. PMID: 20857208
-
[Fetal bovine serum enhances expression of PEDF in epidermal keratinocytes and dermal fibroblasts].Zhejiang Da Xue Xue Bao Yi Xue Ban. 2009 Jul;38(4):343-7. Zhejiang Da Xue Xue Bao Yi Xue Ban. 2009. PMID: 19693969 Chinese.
-
The applied biochemistry of PEDF and implications for tissue homeostasis.Growth Factors. 2010 Aug;28(4):280-5. doi: 10.3109/08977191003604513. Growth Factors. 2010. PMID: 20166889 Free PMC article. Review.
-
Pigment epithelium-derived factor (PEDF) as a therapeutic target in cardiovascular disease.Expert Opin Ther Targets. 2009 Nov;13(11):1295-302. doi: 10.1517/14728220903241641. Expert Opin Ther Targets. 2009. PMID: 19694500 Review.
Cited by
-
Pigment Epithelium-Derived Factor (PEDF) as a Regulator of Wound Angiogenesis.Sci Rep. 2018 Jul 24;8(1):11142. doi: 10.1038/s41598-018-29465-9. Sci Rep. 2018. PMID: 30042381 Free PMC article.
-
The Various Roles of PEDF in Cancer.Cancers (Basel). 2024 Jan 24;16(3):510. doi: 10.3390/cancers16030510. Cancers (Basel). 2024. PMID: 38339261 Free PMC article. Review.
-
Angiogenesis is inhibitory for mammalian digit regeneration.Regeneration (Oxf). 2014 Oct 12;1(3):33-46. doi: 10.1002/reg2.24. eCollection 2014 Jun. Regeneration (Oxf). 2014. PMID: 27499862 Free PMC article.
-
A Narrative Review of Diabetic Macroangiopathy: From Molecular Mechanism to Therapeutic Approaches.Diabetes Ther. 2024 Mar;15(3):585-609. doi: 10.1007/s13300-024-01532-7. Epub 2024 Feb 2. Diabetes Ther. 2024. PMID: 38302838 Free PMC article. Review.
-
Application of quantitative proteomics to discover biomarkers for tick resistance in cattle.Front Immunol. 2023 Jan 30;14:1091066. doi: 10.3389/fimmu.2023.1091066. eCollection 2023. Front Immunol. 2023. PMID: 36793724 Free PMC article.
References
-
- Dawson DW, Volpert OV, Gillis P, et al. Pigment epithelium-derived factor: a potent inhibitor of angiogenesis. Science (New York, NY) 1999;285:245–248. - PubMed
-
- Filleur S, Nelius T, de Riese W, Kennedy RC. Characterization of PEDF: a multi-functional serpin family protein. Journal of cellular biochemistry. 2009;106:769–775. - PubMed
-
- Ek ET, Dass CR, Choong PF. PEDF: a potential molecular therapeutic target with multiple anti-cancer activities. Trends in molecular medicine. 2006;12:497–502. - PubMed
-
- Ek ET, Dass CR, Choong PF. Pigment epithelium-derived factor: a multimodal tumor inhibitor. Molecular cancer therapeutics. 2006;5:1641–1646. - PubMed
-
- Abe R, Yamagishi S, Fujita Y, et al. Topical application of anti-angiogenic peptides based on pigment epithelium-derived factor can improve psoriasis. Journal of dermatological science. 2010;57:183–191. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

