15-deoxy-Δ¹²,¹⁴-PGJ₂ promotes inflammation and apoptosis in cardiomyocytes via the DP2/MAPK/TNFα axis
- PMID: 24698234
- PMCID: PMC4008937
- DOI: 10.1016/j.ijcard.2014.03.086
15-deoxy-Δ¹²,¹⁴-PGJ₂ promotes inflammation and apoptosis in cardiomyocytes via the DP2/MAPK/TNFα axis
Abstract
Background: Prostaglandins (PGs), lipid autacoids derived from arachidonic acid, play a pivotal role during inflammation. PGD₂ synthase is abundantly expressed in heart tissue and PGD₂ has recently been found to induce cardiomyocyte apoptosis. PGD₂ is an unstable prostanoid metabolite; therefore the objective of the present study was to elucidate whether its final dehydration product, 15-deoxy-Δ¹²,¹⁴-PGJ₂ (15d-PGJ₂, present at high levels in ischemic myocardium) might cause cardiomyocyte damage.
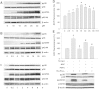
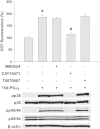
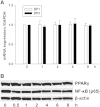
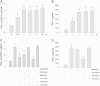
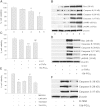
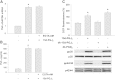
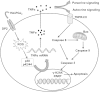
Methods and results: Using specific (ant)agonists we show that 15d-PGJ₂ induced formation of intracellular reactive oxygen species (ROS) and phosphorylation of p38 and p42/44 MAPKs via the PGD2 receptor DP2 (but not DP1 or PPARγ) in the murine atrial cardiomyocyte HL-1 cell line. Activation of the DP2-ROS-MAPK axis by 15d-PGJ₂ enhanced transcription and translation of TNFα and induced apoptosis in HL-1 cardiomyocytes. Silencing of TNFα significantly attenuated the extrinsic (caspase-8) and intrinsic apoptotic pathways (bax and caspase-9), caspase-3 activation and downstream PARP cleavage and γH2AX activation. The apoptotic machinery was unaffected by intracellular calcium, transcription factor NF-κB and its downstream target p53. Of note, 9,10-dihydro-15d-PGJ₂ (lacking the electrophilic carbon atom in the cyclopentenone ring) did not activate cellular responses. Selected experiments performed in primary murine cardiomyocytes confirmed data obtained in HL-1 cells namely that the intrinsic and extrinsic apoptotic cascades are activated via DP2/MAPK/TNFα signaling.
Conclusions: We conclude that the reactive α,β-unsaturated carbonyl group of 15d-PGJ₂ is responsible for the pronounced upregulation of TNFα promoting cardiomyocyte apoptosis. We propose that inhibition of DP2 receptors could provide a possibility to modulate 15d-PGJ₂-induced myocardial injury.
Keywords: 15d-PGJ(2); Apoptosis; Cardiomyocytes; PGD(2) receptor; TNFα.
Copyright © 2014. Published by Elsevier Ireland Ltd.
Figures

Comment in
-
Tsikas et al. 15-deoxy-Δ12,14-PGJ2: An interesting but unapproachable pharmacological target [corrected]?Int J Cardiol. 2014 Nov 15;177(1):307-9. doi: 10.1016/j.ijcard.2014.08.145. Epub 2014 Aug 30. Int J Cardiol. 2014. PMID: 25217216 No abstract available.
-
Response to letter by Tsikas et al.Int J Cardiol. 2014 Nov 15;177(1):140-1. doi: 10.1016/j.ijcard.2014.09.111. Epub 2014 Sep 28. Int J Cardiol. 2014. PMID: 25499360 No abstract available.
References
-
- Agrawal R., Agrawal N., Koyani C.N., Singh R. Molecular targets and regulators of cardiac hypertrophy. Pharmacol Res. 2010;61:269–280. - PubMed
-
- Kalra B.S., Roy V. Efficacy of metabolic modulators in ischemic heart disease: an overview. J Clin Pharmacol. 2012;52:292–305. - PubMed
-
- Dumont E.A., Hofstra L., van Heerde W.L. Cardiomyocyte death induced by myocardial ischemia and reperfusion: measurement with recombinant human annexin-V in a mouse model. Circulation. 2000;102:1564–1568. - PubMed
-
- Ryu S.K., Mallat Z., Benessiano J. Phospholipase A2 enzymes, high-dose atorvastatin, and prediction of ischemic events after acute coronary syndromes. Circulation. 2012;125:757–766. - PubMed
-
- Gross G.J., Falck J.R., Gross E.R., Isbell M., Moore J., Nithipatikom K. Cytochrome P450 and arachidonic acid metabolites: role in myocardial ischemia/reperfusion injury revisited. Cardiovasc Res. 2005;68:18–25. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

