Two Rab2 interactors regulate dense-core vesicle maturation
- PMID: 24698274
- PMCID: PMC3997996
- DOI: 10.1016/j.neuron.2014.02.017
Two Rab2 interactors regulate dense-core vesicle maturation
Abstract
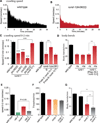
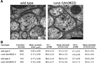
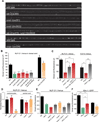
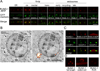
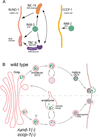
Peptide neuromodulators are released from a unique organelle: the dense-core vesicle. Dense-core vesicles are generated at the trans-Golgi and then sort cargo during maturation before being secreted. To identify proteins that act in this pathway, we performed a genetic screen in Caenorhabditis elegans for mutants defective in dense-core vesicle function. We identified two conserved Rab2-binding proteins: RUND-1, a RUN domain protein, and CCCP-1, a coiled-coil protein. RUND-1 and CCCP-1 colocalize with RAB-2 at the Golgi, and rab-2, rund-1, and cccp-1 mutants have similar defects in sorting soluble and transmembrane dense-core vesicle cargos. RUND-1 also interacts with the Rab2 GAP protein TBC-8 and the BAR domain protein RIC-19, a RAB-2 effector. In summary, a pathway of conserved proteins controls the maturation of dense-core vesicles at the trans-Golgi network.
Copyright © 2014 Elsevier Inc. All rights reserved.
Figures


References
-
- Ann K, Kowalchyk JA, Loyet KM, Martin TF. Novel Ca2+-binding protein (CAPS) related to UNC-31 required for Ca2+-activated exocytosis. J. Biol. Chem. 1997;272:19637–19640. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

