Emergence of context-dependent variability across a basal ganglia network
- PMID: 24698276
- PMCID: PMC4132189
- DOI: 10.1016/j.neuron.2014.01.039
Emergence of context-dependent variability across a basal ganglia network
Abstract
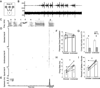
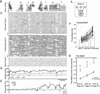
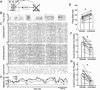
Context dependence is a key feature of cortical-basal ganglia circuit activity, and in songbirds the cortical outflow of a basal ganglia circuit specialized for song, LMAN, shows striking increases in trial-by-trial variability and bursting when birds sing alone rather than to females. To reveal where this variability and its social regulation emerge, we recorded stepwise from corticostriatal (HVC) neurons and their target spiny and pallidal neurons in Area X. We find that corticostriatal and spiny neurons both show precise singing-related firing across both social settings. Pallidal neurons, in contrast, exhibit markedly increased trial-by-trial variation when birds sing alone, created by highly variable pauses in firing. This variability persists even when recurrent inputs from LMAN are ablated. These data indicate that variability and its context sensitivity emerge within the basal ganglia network, suggest a network mechanism for this emergence, and highlight variability generation and regulation as basal ganglia functions.
Copyright © 2014 Elsevier Inc. All rights reserved.
Figures





Comment in
-
Achieving perfection through variability: the basal ganglia helped me do it!Neuron. 2014 Apr 2;82(1):6-8. doi: 10.1016/j.neuron.2014.03.010. Neuron. 2014. PMID: 24698264
References
-
- Bar Gad I, Bergman H. Stepping out of the box: information processing in the neural networks of the basal ganglia. Curr. Opin. Neurobiol. 2001;11:689–695. - PubMed
-
- Bergman H, Feingold A, Nini A, Raz A, Slovin H, Abeles M, Vaadia E. Physiological aspects of information processing in the basal ganglia of normal and parkinsonian primates. TINS. 1998;21:32–38. - PubMed
-
- Bottjer SW, Miesner EA, Arnold AP. Forebrain lesions disrupt development but not maintenance of song in passerine birds. Science. 1984;224:901–903. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

