State dependence of noise correlations in macaque primary visual cortex
- PMID: 24698278
- PMCID: PMC3990250
- DOI: 10.1016/j.neuron.2014.02.006
State dependence of noise correlations in macaque primary visual cortex
Abstract
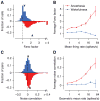
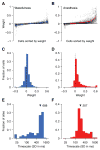
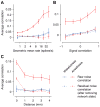
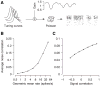
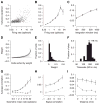
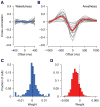
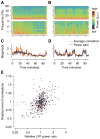
Shared, trial-to-trial variability in neuronal populations has a strong impact on the accuracy of information processing in the brain. Estimates of the level of such noise correlations are diverse, ranging from 0.01 to 0.4, with little consensus on which factors account for these differences. Here we addressed one important factor that varied across studies, asking how anesthesia affects the population activity structure in macaque primary visual cortex. We found that under opioid anesthesia, activity was dominated by strong coordinated fluctuations on a timescale of 1-2 Hz, which were mostly absent in awake, fixating monkeys. Accounting for these global fluctuations markedly reduced correlations under anesthesia, matching those observed during wakefulness and reconciling earlier studies conducted under anesthesia and in awake animals. Our results show that internal signals, such as brain state transitions under anesthesia, can induce noise correlations but can also be estimated and accounted for based on neuronal population activity.
Copyright © 2014 Elsevier Inc. All rights reserved.
Figures


References
-
- Abbott LF, Dayan P. The Effect of Correlated Variability on the Accuracy of a Population Code. Neural Computation. 1999;11(1):91–101. - PubMed
-
- Angel A. Central Neuronal Pathways and the Process of Anaesthesia. British Journal of Anaesthesia. 1993;71(1):148–163. - PubMed
-
- Averbeck BB, Latham PE, Pouget A. Neural correlations, population coding and computation. Nat Rev Neurosci. 2006;7(5):358–366. - PubMed
-
- Bach M, Krüger J. Correlated neuronal variability in monkey visual cortex revealed by a multi-microelectrode. Experimental Brain Research. 1986;61(3):451–456. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

