Neutral polymer micelle carriers with pH-responsive, endosome-releasing activity modulate antigen trafficking to enhance CD8(+) T cell responses
- PMID: 24698946
- PMCID: PMC4156909
- DOI: 10.1016/j.jconrel.2014.03.041
Neutral polymer micelle carriers with pH-responsive, endosome-releasing activity modulate antigen trafficking to enhance CD8(+) T cell responses
Abstract
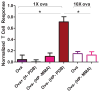
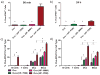
Synthetic subunit vaccines need to induce CD8(+) cytotoxic T cell (CTL) responses for effective vaccination against intracellular pathogens. Most subunit vaccines primarily generate humoral immune responses, with a weaker than desired CD8(+) cytotoxic T cell response. Here, a neutral, pH-responsive polymer micelle carrier that alters intracellular antigen trafficking was shown to enhance CD8(+) T cell responses with a correlated increase in cytosolic delivery and a decrease in exocytosis. Polymer diblock carriers consisted of a N-(2-hydroxypropyl) methacrylamide corona block with pendent pyridyl disulfide groups for reversible conjugation of thiolated ovalbumin, and a tercopolymer ampholytic core-forming block composed of propylacrylic acid (PAA), dimethylaminoethyl methacrylate (DMAEMA), and butyl methacrylate (BMA). The diblock copolymers self-assembled into 25-30nm diameter micellar nanoparticles. Conjugation of ovalbumin to the micelles significantly enhanced antigen cross-presentation in vitro relative to free ovalbumin, an unconjugated physical mixture of ovalbumin and polymer, and a non-pH-responsive micelle-ovalbumin control. Mechanistic studies in a murine dendritic cell line (DC 2.4) demonstrated micelle-mediated enhancements in intracellular antigen retention and cytosolic antigen accumulation. Approximately 90% of initially internalized ovalbumin-conjugated micelles were retained in cells after 1.5h, compared to only ~40% for controls. Furthermore, cells dosed with conjugates displayed 67-fold higher cytosolic antigen levels relative to soluble ovalbumin 4h post uptake. Subcutaneous immunization of mice with ovalbumin-polymer conjugates significantly enhanced antigen-specific CD8(+) T cell responses (0.4% IFN-γ(+) of CD8(+)) compared to immunization with soluble protein, ovalbumin and polymer mixture, and the control micelle without endosome-releasing activity. Additionally, pH-responsive carrier facilitated antigen delivery to antigen presenting cells in the draining lymph nodes. As early as 90min post injection, ova-micelle conjugates were associated with 28% and 55% of dendritic cells and macrophages, respectively. After 24h, conjugates preferentially associated with dendritic cells, affording 30-, 3-, and 3-fold enhancements in uptake relative to free protein, physical mixture, and the non-pH-responsive conjugate controls, respectively. These results demonstrate the potential of pH-responsive polymeric micelles for use in vaccine applications that rely on CD8(+) T cell activation.
Keywords: CD8(+) T cell response; Nanoparticles; Polymer micelles; Subunit vaccine; pH-responsive.
Copyright © 2014 Elsevier B.V. All rights reserved.
Conflict of interest statement
Dr. Stayton is a co-founder of PhaseRx Inc. that has licensed some of the polymer technology represented in this work from the University of Washington. All work described in this report was conducted at the University of Washington independently of PhaseRx Inc. and supported as described in the Acknowledgements.
Figures



Similar articles
-
pH-Responsive nanoparticle vaccines for dual-delivery of antigens and immunostimulatory oligonucleotides.ACS Nano. 2013 May 28;7(5):3912-25. doi: 10.1021/nn305466z. Epub 2013 Apr 30. ACS Nano. 2013. PMID: 23590591 Free PMC article.
-
Antigen delivery with poly(propylacrylic acid) conjugation enhances MHC-1 presentation and T-cell activation.Bioconjug Chem. 2009 Feb;20(2):241-8. doi: 10.1021/bc800317a. Bioconjug Chem. 2009. PMID: 19125614 Free PMC article.
-
Intracellular delivery of a protein antigen with an endosomal-releasing polymer enhances CD8 T-cell production and prophylactic vaccine efficacy.Bioconjug Chem. 2010 Dec 15;21(12):2205-12. doi: 10.1021/bc100204m. Epub 2010 Nov 2. Bioconjug Chem. 2010. PMID: 21043513 Free PMC article.
-
Targeted polymeric micelles for delivery of poorly soluble drugs.Cell Mol Life Sci. 2004 Oct;61(19-20):2549-59. doi: 10.1007/s00018-004-4153-5. Cell Mol Life Sci. 2004. PMID: 15526161 Free PMC article. Review.
-
Biomaterials for nanoparticle vaccine delivery systems.Pharm Res. 2014 Oct;31(10):2563-82. doi: 10.1007/s11095-014-1419-y. Epub 2014 May 22. Pharm Res. 2014. PMID: 24848341 Free PMC article. Review.
Cited by
-
Direct-Ink Write 3D Printing Multistimuli-Responsive Hydrogels and Post-Functionalization Via Disulfide Exchange.ACS Appl Polym Mater. 2022 May 13;4(5):3054-3061. doi: 10.1021/acsapm.1c01538. Epub 2022 Jan 6. ACS Appl Polym Mater. 2022. PMID: 38239328 Free PMC article.
-
Material design for lymph node drug delivery.Nat Rev Mater. 2019 Jun;4(6):415-428. doi: 10.1038/s41578-019-0110-7. Epub 2019 May 2. Nat Rev Mater. 2019. PMID: 32523780 Free PMC article.
-
Guiding principles in the design of molecular bioconjugates for vaccine applications.Bioconjug Chem. 2015 May 20;26(5):791-801. doi: 10.1021/acs.bioconjchem.5b00103. Epub 2015 Apr 16. Bioconjug Chem. 2015. PMID: 25822926 Free PMC article. Review.
-
pH-Responsive Amphiphilic Carboxylate Polymers: Design and Potential for Endosomal Escape.Front Chem. 2021 Mar 23;9:645297. doi: 10.3389/fchem.2021.645297. eCollection 2021. Front Chem. 2021. PMID: 33834015 Free PMC article. Review.
-
Biomaterials for vaccine-based cancer immunotherapy.J Control Release. 2018 Dec 28;292:256-276. doi: 10.1016/j.jconrel.2018.10.008. Epub 2018 Oct 9. J Control Release. 2018. PMID: 30312721 Free PMC article. Review.
References
-
- Swartz MA, Hirosue S, Hubbell JA. Engineering approaches to immunotherapy. Sci Transl Med. 2012;4:148rv9–148rv9. - PubMed
-
- Hubbell JA, Thomas SN, Swartz MA. Materials engineering for immunomodulation. Nature. 2009;462:449–60. - PubMed
-
- De Temmerman ML, Rejman J, Demeester J, Irvine DJ, Gander B, De Smedt SC. Particulate vaccines: on the quest for optimal delivery and immune response. Drug Discov Today. 2011;16:569–82. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

