Effect of the premalignant and tumor microenvironment on immune cell cytokine production in head and neck cancer
- PMID: 24698959
- PMCID: PMC4074802
- DOI: 10.3390/cancers6020756
Effect of the premalignant and tumor microenvironment on immune cell cytokine production in head and neck cancer
Abstract
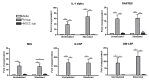
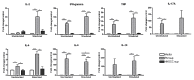
Head and neck squamous cell carcinoma (HNSCC) is marked by immunosuppression, a state in which the established tumor escapes immune attack. However, the impact of the premalignant and tumor microenvironments on immune reactivity has yet to be elucidated. The purpose of this study was to determine how soluble mediators from cells established from carcinogen-induced oral premalignant lesions and HNSCC modulate immune cell cytokine production. It was found that premalignant cells secrete significantly increased levels of G-CSF, RANTES, MCP-1, and PGE2 compared to HNSCC cells. Splenocytes incubated with premalignant supernatant secreted significantly increased levels of Th1-, Th2-, and Th17-associated cytokines compared to splenocytes incubated with HNSCC supernatant. These studies demonstrate that whereas the premalignant microenvironment elicits proinflammatory cytokine production, the tumor microenvironment is significantly less immune stimulatory and may contribute to immunosuppression in established HNSCC.
Figures

References
-
- Bergmann C., Strauss L., Wang Y., Szczepanski M.J., Lang S., Johnson J.T., Whiteside T.L. T regulatory type I cells in squamous cell carcinoma of the head and neck: Mechanisms of suppression and expansion in advanced disease. Clin. Cancer Res. 2008;14:3706–3715. doi: 10.1158/1078-0432.CCR-07-5126. - DOI - PMC - PubMed
-
- Badoual C., Hans S., Rodriguez J., Peyrard S., Klein C., Agueznay Nel H., Mosseri V., Laccourreye O., Bruneval P., Fridman W.H., et al. Prognostic value of tumor-infiltrating CD4+ T-cell subpopulations in head and neck cancers. Clin. Cancer Res. 2006;12:465–472. doi: 10.1158/1078-0432.CCR-05-1886. - DOI - PubMed
-
- Young M.R., Wright M.A., Lozano Y., Prechel M.M., Benefield J., Leonetti J.P., Collins S.L., Petruzzelli G.J. Increased recurrence and metastasis in patients whose primary head and neck squamous cell carcinomas secreted granulocyte-macrophage colony-stimulating factor and contained CD34+ natural suppressor cells. Int. J. Cancer. 1997;74:69–74. doi: 10.1002/(SICI)1097-0215(19970220)74:1<69::AID-IJC12>3.0.CO;2-D. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

