Clinical and pathologic findings of Spitz nevi and atypical Spitz tumors with ALK fusions
- PMID: 24698967
- PMCID: PMC5042334
- DOI: 10.1097/PAS.0000000000000187
Clinical and pathologic findings of Spitz nevi and atypical Spitz tumors with ALK fusions
Abstract
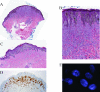
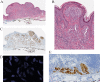
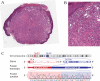
Spitz tumors represent a group of melanocytic neoplasms that typically affect young individuals. Microscopically, the lesions are composed of cytologically distinct spindle and epithelioid melanocytes, with a range in the architectural display or the cells, their nuclear features, and secondary epidermal or stromal changes. Recently, kinase fusions have been documented in a subset of Spitz tumors, but there is limited information on the clinical and pathologic features associated with those lesions. Here, we report a series of 17 patients (9 male, 8 female) with spitzoid neoplasms showing ALK fusions (5 Spitz nevi and 12 atypical Spitz tumors). The patients' ages ranged from 2 years to 35 years (mean=17 y; median=16 y). Most lesions were located on the lower extremities and presented clinically as polypoid nodules. All tumors were compound melanocytic proliferations with a predominant intradermal growth. Tumor thickness ranged from 1.1 to 6 mm (mean=2.9 mm; median=2.5 mm). The most characteristic histopathologic feature of the tumors (seen in all but 2 lesions) was a plexiform dermal growth of intersecting fascicles of fusiform melanocytes. All but 2 tumors were amelanotic. All tumors were strongly immunoreactive for ALK. The ALK rearrangements were confirmed in all cases by fluorescence in situ hybridization (FISH), and the fusion partner was determined by quantitative polymerase chain reaction as TPM3 (tropomyosin 3) in 11 cases and DCTN1 (dynactin 1) in 6 cases. None of the 8 tumors that were analyzed by FISH for copy number changes of 6p, 6q, 9p, or 11q met criteria for melanoma. Two patients underwent a sentinel lymph node biopsy, and in both cases melanocyte nests were found in the subcapsular sinus of the node. Array comparative genomic hybridization of these 2 tumors revealed no chromosomal gains or losses. In conclusion, our study revealed that Spitz nevi/tumors with ALK rearrangement show a characteristic plexiform morphology and that ALK immunohistochemistry and FISH enable the accurate identification of this morphologic and genetic distinct subset of spitzoid neoplasms.
Figures



References
-
- Zedek DC, McCalmont TH. Spitz nevi, atypical spitzoid neoplasms, and spitzoid melanoma. Clin Lab Med. 2011;31(2):311–20. - PubMed
-
- Bastian BC, et al. Molecular cytogenetic analysis of Spitz nevi shows clear differences to melanoma. J Invest Dermatol. 1999;113(6):1065–9. - PubMed
-
- Fullen DR, et al. BRAF and NRAS mutations in spitzoid melanocytic lesions. Mod Pathol. 2006;19(10):1324–32. - PubMed
-
- Gerami P, Busam KJ. Cytogenetic and mutational analyses of melanocytic tumors. Dermatol Clin. 2012;30(4):555–66. v. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

