Dipolar Assisted Assignment Protocol (DAAP) for MAS solid-state NMR of rotationally aligned membrane proteins in phospholipid bilayers
- PMID: 24698983
- PMCID: PMC4043445
- DOI: 10.1016/j.jmr.2014.02.018
Dipolar Assisted Assignment Protocol (DAAP) for MAS solid-state NMR of rotationally aligned membrane proteins in phospholipid bilayers
Abstract
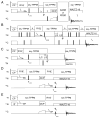
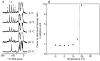
A method for making resonance assignments in magic angle spinning solid-state NMR spectra of membrane proteins that utilizes the range of heteronuclear dipolar coupling frequencies in combination with conventional chemical shift based assignment methods is demonstrated. The Dipolar Assisted Assignment Protocol (DAAP) takes advantage of the rotational alignment of the membrane proteins in liquid crystalline phospholipid bilayers. Improved resolution is obtained by combining the magnetically inequivalent heteronuclear dipolar frequencies with isotropic chemical shift frequencies. Spectra with both dipolar and chemical shift frequency axes assist with resonance assignments. DAAP can be readily extended to three- and four-dimensional experiments and to include both backbone and side chain sites in proteins.
Keywords: MAS; Membrane protein; Solid state NMR; Vpu.
Copyright © 2014 Elsevier Inc. All rights reserved.
Figures






References
-
- McDermott A. Structure and dynamics of membrane proteins by magic angle spinning solid-state NMR. Annu Rev Biophys. 2009;38:385–403. - PubMed
-
- Schuetz A, Wasmer C, Habenstein B, Verel R, Greenwald J, Riek R, Böckmann A, Meier BH. Protocols for the Sequential Solid-State NMR Spectroscopic Assignment of a Uniformly Labeled 25 kDa Protein: HET-s(1–227) ChemBioChem. 2010;11:1543–1551. - PubMed
-
- Shi L, Lake EM, Ahmed MA, Brown LS, Ladizhansky V. Solid-state NMR study of proteorhodopsin in the lipid environment: secondary structure and dynamics. Biochimica et Biophysica Acta (BBA)-Biomembranes. 2009;1788:2563–2574. - PubMed
-
- Tang W, Knox RW, Nevzorov AA. A spectroscopic assignment technique for membrane proteins reconstituted in magnetically aligned bicelles. J Biomol NMR. 2012;54:307–316. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

