Colonic inflammation and secondary bile acids in alcoholic cirrhosis
- PMID: 24699327
- PMCID: PMC4152166
- DOI: 10.1152/ajpgi.00315.2013
Colonic inflammation and secondary bile acids in alcoholic cirrhosis
Abstract
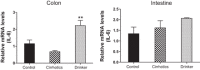
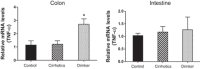
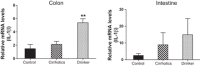
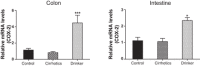
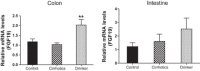
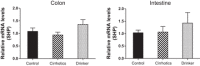
Alcohol abuse with/without cirrhosis is associated with an impaired gut barrier and inflammation. Gut microbiota can transform primary bile acids (BA) to secondary BAs, which can adversely impact the gut barrier. The purpose of this study was to define the effect of active alcohol intake on fecal BA levels and ileal and colonic inflammation in cirrhosis. Five age-matched groups {two noncirrhotic (control and drinkers) and three cirrhotic [nondrinkers/nonalcoholics (NAlc), abstinent alcoholic for >3 mo (AbsAlc), currently drinking (CurrAlc)]} were included. Fecal and serum BA analysis, serum endotoxin, and stool microbiota using pyrosequencing were performed. A subgroup of controls, NAlc, and CurrAlc underwent ileal and sigmoid colonic biopsies on which mRNA expression of TNF-α, IL-1β, IL-6, and cyclooxygenase-2 (Cox-2) were performed. One hundred three patients (19 healthy, 6 noncirrhotic drinkers, 10 CurrAlc, 38 AbsAlc, and 30 NAlc, age 56 yr, median MELD: 10.5) were included. Five each of healthy, CurrAlc, and NAlc underwent ileal/colonic biopsies. Endotoxin, serum-conjugated DCA and stool total BAs, and secondary-to-primary BA ratios were highest in current drinkers. On biopsies, a significantly higher mRNA expression of TNF-α, IL-1β, IL-6, and Cox-2 in colon but not ileum was seen in CurrAlc compared with NAlc and controls. Active alcohol use in cirrhosis is associated with a significant increase in the secondary BA formation compared with abstinent alcoholic cirrhotics and nonalcoholic cirrhotics. This increase in secondary BAs is associated with a significant increase in expression of inflammatory cytokines in colonic mucosa but not ileal mucosa, which may contribute to alcohol-induced gut barrier injury.
Keywords: alcohol; cytokines; farnesoid X receptor; microbiome.
Copyright © 2014 the American Physiological Society.
Figures


Similar articles
-
Continued Alcohol Misuse in Human Cirrhosis is Associated with an Impaired Gut-Liver Axis.Alcohol Clin Exp Res. 2017 Nov;41(11):1857-1865. doi: 10.1111/acer.13498. Epub 2017 Oct 11. Alcohol Clin Exp Res. 2017. PMID: 28925102
-
Modulation of the fecal bile acid profile by gut microbiota in cirrhosis.J Hepatol. 2013 May;58(5):949-55. doi: 10.1016/j.jhep.2013.01.003. Epub 2013 Jan 16. J Hepatol. 2013. PMID: 23333527 Free PMC article.
-
Altered Microbiota Diversity and Bile Acid Signaling in Cirrhotic and Noncirrhotic NASH-HCC.Clin Transl Gastroenterol. 2020 Mar;11(3):e00131. doi: 10.14309/ctg.0000000000000131. Clin Transl Gastroenterol. 2020. PMID: 32352707 Free PMC article.
-
Comprehensive Analysis of Serum and Fecal Bile Acid Profiles and Interaction with Gut Microbiota in Primary Biliary Cholangitis.Clin Rev Allergy Immunol. 2020 Feb;58(1):25-38. doi: 10.1007/s12016-019-08731-2. Clin Rev Allergy Immunol. 2020. PMID: 30900136
-
Secondary Bile Acids and Short Chain Fatty Acids in the Colon: A Focus on Colonic Microbiome, Cell Proliferation, Inflammation, and Cancer.Int J Mol Sci. 2019 Mar 11;20(5):1214. doi: 10.3390/ijms20051214. Int J Mol Sci. 2019. PMID: 30862015 Free PMC article. Review.
Cited by
-
The role of the gut-brain axis in alcohol use disorders.Prog Neuropsychopharmacol Biol Psychiatry. 2016 Feb 4;65:234-41. doi: 10.1016/j.pnpbp.2015.06.013. Epub 2015 Jul 16. Prog Neuropsychopharmacol Biol Psychiatry. 2016. PMID: 26188287 Free PMC article. Review.
-
Alcoholic liver disease: the gut microbiome and liver cross talk.Alcohol Clin Exp Res. 2015 May;39(5):763-75. doi: 10.1111/acer.12704. Alcohol Clin Exp Res. 2015. PMID: 25872593 Free PMC article. Review.
-
Liver cirrhosis and complications from the perspective of dysbiosis.Front Med (Lausanne). 2024 Jan 16;10:1320015. doi: 10.3389/fmed.2023.1320015. eCollection 2023. Front Med (Lausanne). 2024. PMID: 38293307 Free PMC article. Review.
-
Deregulated neddylation in liver fibrosis.Hepatology. 2017 Feb;65(2):694-709. doi: 10.1002/hep.28933. Epub 2016 Dec 30. Hepatology. 2017. PMID: 28035772 Free PMC article.
-
Gut microbiome changes in Nonalcoholic fatty liver disease & alcoholic liver disease.Transl Gastroenterol Hepatol. 2021 Jan 5;6:3. doi: 10.21037/tgh.2020.02.18. eCollection 2021. Transl Gastroenterol Hepatol. 2021. PMID: 33409398 Free PMC article. Review.
References
-
- Adachi Y, Moore LE, Bradford BU, Gao W, Thurman RG. Antibiotics prevent liver injury in rats following long-term exposure to ethanol. Gastroenterology 108: 218–224, 1995 - PubMed
-
- Axelson M, Mork B, Sjovall J. Ethanol has an acute effect on bile acid biosynthesis in man. FEBS Lett 281: 155–159, 1991 - PubMed
-
- Bajaj JS, Hylemon PB, Ridlon JM, Heuman DM, Daita K, White MB, Monteith P, Noble NA, Sikaroodi M, Gillevet PM. The colonic mucosal microbiome differs from stool microbiome in cirrhosis and hepatic encephalopathy and is linked to cognition and inflammation. Am J Physiol Gastrointest Liver Physiol 306: G675–G685, 2014 - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

