Porphyrin-phospholipid liposomes permeabilized by near-infrared light
- PMID: 24699423
- PMCID: PMC3988818
- DOI: 10.1038/ncomms4546
Porphyrin-phospholipid liposomes permeabilized by near-infrared light
Abstract
The delivery of therapeutic compounds to target tissues is a central challenge in treating disease. Externally controlled drug release systems hold potential to selectively enhance localized delivery. Here we describe liposomes doped with porphyrin-phospholipid that are permeabilized directly by near-infrared light. Molecular dynamics simulations identified a novel light-absorbing monomer esterified from clinically approved components predicted and experimentally demonstrated to give rise to a more stable porphyrin bilayer. Light-induced membrane permeabilization is enabled with liposomal inclusion of 10 molar % porphyrin-phospholipid and occurs in the absence of bulk or nanoscale heating. Liposomes reseal following laser exposure and permeability is modulated by varying porphyrin-phospholipid doping, irradiation intensity or irradiation duration. Porphyrin-phospholipid liposomes demonstrate spatial control of release of entrapped gentamicin and temporal control of release of entrapped fluorophores following intratumoral injection. Following systemic administration, laser irradiation enhances deposition of actively loaded doxorubicin in mouse xenografts, enabling an effective single-treatment antitumour therapy.
Figures


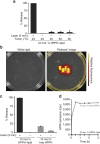

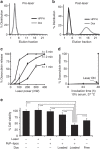
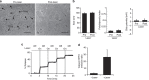

References
-
- Peer D. et al. Nanocarriers as an emerging platform for cancer therapy. Nat. Nanotech. 2, 751–760 (2007). - PubMed
-
- Allen T. M. & Cullis P. R. Liposomal drug delivery systems: from concept to clinical applications. Adv. Drug Deliv. Rev. 65, 36–48 (2013). - PubMed
-
- Li S.-D. & Huang L. Pharmacokinetics and biodistribution of nanoparticles. Mol. Pharm. 5, 496–504 (2008). - PubMed
-
- Timko B. P., Dvir T. & Kohane D. S. Remotely triggerable drug delivery systems. Adv. Mater. 22, 4925–4943 (2010). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

