Antiviral strategies against influenza virus: towards new therapeutic approaches
- PMID: 24699705
- PMCID: PMC11114059
- DOI: 10.1007/s00018-014-1615-2
Antiviral strategies against influenza virus: towards new therapeutic approaches
Abstract
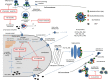
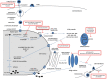
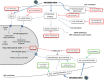
Influenza viruses are major human pathogens responsible for respiratory diseases affecting millions of people worldwide and characterized by high morbidity and significant mortality. Influenza infections can be controlled by vaccination and antiviral drugs. However, vaccines need annual updating and give limited protection. Only two classes of drugs are currently approved for the treatment of influenza: M2 ion channel blockers and neuraminidase inhibitors. However, they are often associated with limited efficacy and adverse side effects. In addition, the currently available drugs suffer from rapid and extensive emergence of drug resistance. All this highlights the urgent need for developing new antiviral strategies with novel mechanisms of action and with reduced drug resistance potential. Several new classes of antiviral agents targeting viral replication mechanisms or cellular proteins/processes are under development. This review gives an overview of novel strategies targeting the virus and/or the host cell for counteracting influenza virus infection.
Figures
References
-
- Cox NJ, Subbarao K. Global epidemiology of influenza: past and present. Annu Rev Med. 2000;51:407–421. - PubMed
-
- Shaw ML, Palese P (2013) Orthomyxoviruses. In: Knipe DM, Howley PM (eds) Fields virology, 6th edn. Lippincott Williams & Wilkins, Philadelphia, pp 1648–1698
-
- Chen W, Calvo PA, Malide D, Gibbs J, Schubert U, Bacik I, Basta S, O’Neill R, Schickli J, Palese P, Henklein P, Bennink JR, Yewdell JW. A novel influenza A virus mitochondrial protein that induces cell death. Nat Med. 2001;7(12):1306–1312. - PubMed
-
- Jagger BW, Wise HM, Kash JC, Walters KA, Wills NM, Xiao YL, Dunfee RL, Schwartzman LM, Ozinsky A, Bell GL, Dalton RM, Lo A, Efstathiou S, Atkins JF, Firth AE, Taubenberger JK, Digard P. An overlapping protein-coding region in influenza A virus segment 3 modulates the host response. Science. 2012;337(6091):199–204. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

