Recombinant bovine respiratory syncytial virus with deletion of the SH gene induces increased apoptosis and pro-inflammatory cytokines in vitro, and is attenuated and induces protective immunity in calves
- PMID: 24700100
- PMCID: PMC4027036
- DOI: 10.1099/vir.0.064931-0
Recombinant bovine respiratory syncytial virus with deletion of the SH gene induces increased apoptosis and pro-inflammatory cytokines in vitro, and is attenuated and induces protective immunity in calves
Abstract
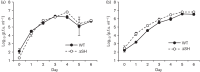
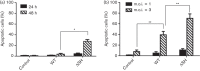
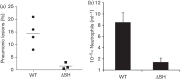
Bovine respiratory syncytial virus (BRSV) causes inflammation and obstruction of the small airways, leading to severe respiratory disease in young calves. The virus is closely related to human (H)RSV, a major cause of bronchiolitis and pneumonia in young children. The ability to manipulate the genome of RSV has provided opportunities for the development of stable, live attenuated RSV vaccines. The role of the SH protein in the pathogenesis of BRSV was evaluated in vitro and in vivo using a recombinant (r)BRSV in which the SH gene had been deleted. Infection of bovine epithelial cells and monocytes with rBRSVΔSH, in vitro, resulted in an increase in apoptosis, and higher levels of TNF-α and IL-1β compared with cells infected with parental, wild-type (WT) rBRSV. Although replication of rBRSVΔSH and WT rBRSV, in vitro, were similar, the replication of rBRSVΔSH was moderately reduced in the lower, but not the upper, respiratory tract of experimentally infected calves. Despite the greater ability of rBRSVΔSH to induce pro-inflammatory cytokines, in vitro, the pulmonary inflammatory response in rBRSVΔSH-infected calves was significantly reduced compared with that in calves inoculated with WT rBRSV, 6 days previously. Virus lacking SH appeared to be as immunogenic and effective in inducing resistance to virulent virus challenge, 6 months later, as the parental rBRSV. These findings suggest that rBRSVΔSH may be an ideal live attenuated virus vaccine candidate, combining safety with a high level of immunogenicity.
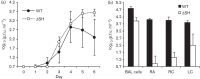
Figures
Similar articles
-
Modulation of the transcription factor NF-κB in antigen-presenting cells by bovine respiratory syncytial virus small hydrophobic protein.J Gen Virol. 2017 Jul;98(7):1587-1599. doi: 10.1099/jgv.0.000855. Epub 2017 Jul 17. J Gen Virol. 2017. PMID: 28714847 Free PMC article.
-
Mucosal immunization with live recombinant bovine respiratory syncytial virus (BRSV) and recombinant BRSV lacking the envelope glycoprotein G protects against challenge with wild-type BRSV.J Virol. 2002 Dec;76(23):12355-9. doi: 10.1128/jvi.76.23.12355-12359.2002. J Virol. 2002. PMID: 12414977 Free PMC article.
-
Bovine respiratory syncytial virus lacking the virokinin or with a mutation in furin cleavage site RA(R/K)R109 induces less pulmonary inflammation without impeding the induction of protective immunity in calves.J Gen Virol. 2006 Jun;87(Pt 6):1659-1667. doi: 10.1099/vir.0.81755-0. J Gen Virol. 2006. PMID: 16690931
-
Bovine respiratory syncytial virus infection.Vet Res. 2007 Mar-Apr;38(2):153-80. doi: 10.1051/vetres:2006053. Epub 2007 Jan 25. Vet Res. 2007. PMID: 17257568 Review.
-
Immunology of bovine respiratory syncytial virus in calves.Mol Immunol. 2015 Jul;66(1):48-56. doi: 10.1016/j.molimm.2014.12.004. Epub 2014 Dec 29. Mol Immunol. 2015. PMID: 25553595 Review.
Cited by
-
The Bacterial and Viral Agents of BRDC: Immune Evasion and Vaccine Developments.Vaccines (Basel). 2021 Apr 1;9(4):337. doi: 10.3390/vaccines9040337. Vaccines (Basel). 2021. PMID: 33916119 Free PMC article. Review.
-
Circulation of Indigenous Bovine Respiratory Syncytial Virus Strains in Turkish Cattle: The First Isolation and Molecular Characterization.Animals (Basel). 2020 Sep 20;10(9):1700. doi: 10.3390/ani10091700. Animals (Basel). 2020. PMID: 32962234 Free PMC article.
-
Review on bovine respiratory syncytial virus and bovine parainfluenza - usual suspects in bovine respiratory disease - a narrative review.BMC Vet Res. 2021 Jul 31;17(1):261. doi: 10.1186/s12917-021-02935-5. BMC Vet Res. 2021. PMID: 34332574 Free PMC article. Review.
-
Mumps Virus SH Protein Inhibits NF-κB Activation by Interacting with Tumor Necrosis Factor Receptor 1, Interleukin-1 Receptor 1, and Toll-Like Receptor 3 Complexes.J Virol. 2017 Aug 24;91(18):e01037-17. doi: 10.1128/JVI.01037-17. Print 2017 Sep 15. J Virol. 2017. PMID: 28659487 Free PMC article.
-
Vaccine safety and efficacy evaluation of a recombinant bovine respiratory syncytial virus (BRSV) with deletion of the SH gene and subunit vaccines based on recombinant human RSV proteins: N-nanorings, P and M2-1, in calves with maternal antibodies.PLoS One. 2014 Jun 19;9(6):e100392. doi: 10.1371/journal.pone.0100392. eCollection 2014. PLoS One. 2014. PMID: 24945377 Free PMC article.
References
-
- Bermejo-Martin J. F., Garcia-Arevalo M. C., Alonso A., De Lejarazu R. O., Pino M., Resino S., Tenorio A., Bernardo D., Leon A. J. & other authors (2007). Persistence of proinflammatory response after severe respiratory syncytial virus disease in children. J Allergy Clin Immunol 119, 1547–1550 10.1016/j.jaci.2007.03.014 - DOI - PubMed
-
- Bukreyev A., Whitehead S. S., Murphy B. R., Collins P. L. (1997). Recombinant respiratory syncytial virus from which the entire SH gene has been deleted grows efficiently in cell culture and exhibits site-specific attenuation in the respiratory tract of the mouse. J Virol 71, 8973–8982 - PMC - PubMed
-
- Carter S. D., Dent K. C., Atkins E., Foster T. L., Verow M., Gorny P., Harris M., Hiscox J. A., Ranson N. A. & other authors (2010). Direct visualization of the small hydrophobic protein of human respiratory syncytial virus reveals the structural basis for membrane permeability. FEBS Lett 584, 2786–2790 10.1016/j.febslet.2010.05.006 - DOI - PMC - PubMed
-
- Dennis M. J., Davies D. C., Hoare M. N. (1976). A simplified apparatus for the microbiological isolation of calves. Br Vet J 132, 642–646 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- BBS/E/I/00001052/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
- BBS/E/I/00001325/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
- BBS/E/I/00001709/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
- ImNIH/Intramural NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources

