Changes in individual drug-independent system parameters during virtual paediatric pharmacokinetic trials: introducing time-varying physiology into a paediatric PBPK model
- PMID: 24700271
- PMCID: PMC4012043
- DOI: 10.1208/s12248-014-9592-9
Changes in individual drug-independent system parameters during virtual paediatric pharmacokinetic trials: introducing time-varying physiology into a paediatric PBPK model
Abstract
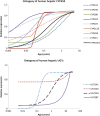
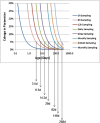
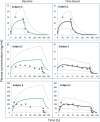
Although both POPPK and physiologically based pharmacokinetic (PBPK) models can account for age and other covariates within a paediatric population, they generally do not account for real-time growth and maturation of the individuals through the time course of drug exposure; this may be significant in prolonged neonatal studies. The major objective of this study was to introduce age progression into a paediatric PBPK model, to allow for continuous updating of anatomical, physiological and biological processes in each individual subject over time. The Simcyp paediatric PBPK model simulator system parameters were reanalysed to assess the impact of re-defining the individual over the study period. A schedule for re-defining parameters within the Simcyp paediatric simulator, for each subject, over a prolonged study period, was devised to allow seamless prediction of pharmacokinetics (PK). The model was applied to predict concentration-time data from multiday studies on sildenafil and phenytoin performed in neonates. Among PBPK system parameters, CYP3A4 abundance was one of the fastest changing covariates and a 1-h re-sampling schedule was needed for babies below age 3.5 days in order to seamlessly predict PK (<5% change in abundance) with subject maturation. The re-sampling frequency decreased as age increased, reaching biweekly by 6 months of age. The PK of both sildenafil and phenytoin were predicted better at the end of a prolonged study period using the time varying vs fixed PBPK models. Paediatric PBPK models which account for time-varying system parameters during prolonged studies may provide more mechanistic PK predictions in neonates and infants.
Figures



References
-
- Abduljalil K, Furness P, Johnson TN, Rostami-Hodjegan A, Soltani H. Anatomical, physiological and metabolic changes with gestational age during normal pregnancy: a database for parameters required in physiologically based pharmacokinetic modelling. Clin Pharmacokinet. 2012;51(6):365–396. doi: 10.2165/11597440-000000000-00000. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

