LIN28 expression in rat spinal cord after injury
- PMID: 24700281
- PMCID: PMC4000414
- DOI: 10.1007/s11064-014-1278-2
LIN28 expression in rat spinal cord after injury
Abstract
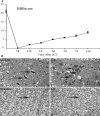
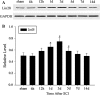
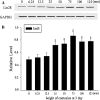
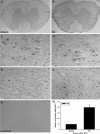
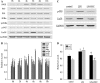
LIN28, an RNA-binding protein, is known to be involved in the regulation of many cellular processes, such as embryonic stem cell proliferation, cell fate succession, developmental timing, and oncogenesis. However, its expression and function in central nervous system still unclear. In this study, we performed an acute spinal cord contusion injury (SCI) model in adult rats and investigated the dynamic changes of LIN28 expression in spinal cord. Western blot and immunohistochemistry analysis revealed that LIN28 was present in normal spinal cord. It gradually increased, reached a peak at 3 day, and then nearly declined to the basal level at 14 days after SCI. Double immunofluorescence staining showed that LIN28 immunoreactivity was found in neurons, astrocytes and a handful of microglia. Interestingly, LIN28 expression was increased predominantly in astrocytes but not in neurons. Moreover, the colocalization of LIN28 and proliferating cell nuclear antigen was detected after injury. Western blot showed that LIN28 participated in lipopolysaccharide (LPS) induced astrocytes inflammatory responses by NF-κB signaling pathway. These results suggested that LIN28 may be involved in the pathologic process of SCI, and further research is needed to have a good understanding of its function and mechanism.
Figures


Similar articles
-
Spatiotemporal profile and essential role of RBM3 expression after spinal cord injury in adult rats.J Mol Neurosci. 2014;54(2):252-63. doi: 10.1007/s12031-014-0282-y. Epub 2014 Mar 26. J Mol Neurosci. 2014. PMID: 24668366
-
Expression of RBMX after spinal cord injury in rats.J Mol Neurosci. 2013 Feb;49(2):417-29. doi: 10.1007/s12031-012-9914-2. Epub 2012 Nov 22. J Mol Neurosci. 2013. PMID: 23180094
-
The upregulation of annexin A2 after spinal cord injury in rats may have implication for astrocyte proliferation.Neuropeptides. 2017 Feb;61:67-76. doi: 10.1016/j.npep.2016.10.007. Epub 2016 Oct 27. Neuropeptides. 2017. PMID: 27836325
-
AKR1B1 Upregulation Contributes to Neuroinflammation and Astrocytes Proliferation by Regulating the Energy Metabolism in Rat Spinal Cord Injury.Neurochem Res. 2018 Aug;43(8):1491-1499. doi: 10.1007/s11064-018-2570-3. Epub 2018 Jun 12. Neurochem Res. 2018. PMID: 29948725 Review.
-
The Role of Microglia in Modulating Neuroinflammation after Spinal Cord Injury.Int J Mol Sci. 2021 Sep 8;22(18):9706. doi: 10.3390/ijms22189706. Int J Mol Sci. 2021. PMID: 34575871 Free PMC article. Review.
Cited by
-
Nephron-Specific Lin28A Overexpression Triggers Severe Inflammatory Response and Kidney Damage.Int J Biol Sci. 2024 Jul 22;20(10):4044-4054. doi: 10.7150/ijbs.97434. eCollection 2024. Int J Biol Sci. 2024. PMID: 39113694 Free PMC article.
-
Blockade on Lin28a Prevents Cognitive Impairment and Disruption of the Blood-Brain Barrier Induced by Chronic Cerebral Hypoperfusion.Biomedicines. 2022 Apr 5;10(4):852. doi: 10.3390/biomedicines10040852. Biomedicines. 2022. PMID: 35453602 Free PMC article.
-
Drinking desalinated seawater for a long time induces anomalies in the development of new-born albino rats.Saudi J Biol Sci. 2017 Sep;24(6):1306-1321. doi: 10.1016/j.sjbs.2016.08.005. Epub 2016 Aug 28. Saudi J Biol Sci. 2017. PMID: 28855826 Free PMC article.
-
Lin28 is associated with astrocytic proliferation during intracerebral hemorrhage.Int J Clin Exp Pathol. 2020 May 1;13(5):1136-1145. eCollection 2020. Int J Clin Exp Pathol. 2020. PMID: 32509088 Free PMC article.
-
LIN28A alleviates inflammation, oxidative stress, osteogenic differentiation and mineralization in lipopolysaccharide (LPS)-treated human periodontal ligament stem cells.Exp Ther Med. 2022 Jun;23(6):411. doi: 10.3892/etm.2022.11338. Epub 2022 Apr 27. Exp Ther Med. 2022. PMID: 35601075 Free PMC article.
References
-
- Saadoun S, Bell BA, Verkman AS, Papadopoulos MC. Greatly improved neurological outcome after spinal cord compression injury in AQP4-deficient mice. Brain. 2008;131(Pt 4):1087–1098. - PubMed
-
- Ceruti S, Villa G, Genovese T, Mazzon E, Longhi R, Rosa P, Bramanti P, Cuzzocrea S, Abbracchio MP. The P2Y-like receptor GPR17 as a sensor of damage and a new potential target in spinal cord injury. Brain. 2009;132(Pt 8):2206–2218. - PubMed
-
- Dumont RJ, Okonkwo DO, Verma S, Hurlbert RJ, Boulos PT, Ellegala DB, Dumont AS. Acute spinal cord injury, part I: pathophysiologic mechanisms. Clin Neuropharmacol. 2001;24(5):254–264. - PubMed
-
- Liu Y, Figley S, Spratt SK, Lee G, Ando D, Surosky R, Fehlings MG. An engineered transcription factor which activates VEGF-A enhances recovery after spinal cord injury. Neurobiol Dis. 2010;37(2):384–393. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

