Gain-of-function mutation in Gnao1: a murine model of epileptiform encephalopathy (EIEE17)?
- PMID: 24700286
- PMCID: PMC4042023
- DOI: 10.1007/s00335-014-9509-z
Gain-of-function mutation in Gnao1: a murine model of epileptiform encephalopathy (EIEE17)?
Abstract
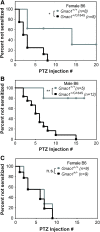
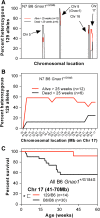
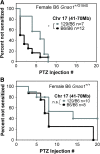
G protein-coupled receptors strongly modulate neuronal excitability but there has been little evidence for G protein mechanisms in genetic epilepsies. Recently, four patients with epileptic encephalopathy (EIEE17) were found to have mutations in GNAO1, the most abundant G protein in brain, but the mechanism of this effect is not known. The GNAO1 gene product, Gαo, negatively regulates neurotransmitter release. Here, we report a dominant murine model of Gnao1-related seizures and sudden death. We introduced a genomic gain-of-function knock-in mutation (Gnao1 (+/G184S)) that prevents Go turnoff by Regulators of G protein signaling proteins. This results in rare seizures, strain-dependent death between 15 and 40 weeks of age, and a markedly increased frequency of interictal epileptiform discharges. Mutants on a C57BL/6J background also have faster sensitization to pentylenetetrazol (PTZ) kindling. Both premature lethality and PTZ kindling effects are suppressed in the 129SvJ mouse strain. We have mapped a 129S-derived modifier locus on Chromosome 17 (within the region 41-70 MB) as a Modifer of G protein Seizures (Mogs1). Our mouse model suggests a novel gain-of-function mechanism for the newly defined subset of epileptic encephalopathy (EIEE17). Furthermore, it reveals a new epilepsy susceptibility modifier Mogs1 with implications for the complex genetics of human epilepsy as well as sudden death in epilepsy.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

