Clonal tracing of Sox9+ liver progenitors in mouse oval cell injury
- PMID: 24700457
- PMCID: PMC4077948
- DOI: 10.1002/hep.27084
Clonal tracing of Sox9+ liver progenitors in mouse oval cell injury
Abstract
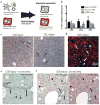
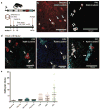
Proliferating ducts, termed "oval cells," have long been thought to be bipotential, that is, produce both biliary ducts and hepatocytes during chronic liver injury. The precursor to oval cells is considered to be a facultative liver stem cell (LSC). Recent lineage tracing experiments indicated that the LSC is SRY-related HMG box transcription factor 9 positive (Sox9(+) ) and can replace the bulk of hepatocyte mass in several settings. However, no clonal relationship between Sox9(+) cells and the two epithelial liver lineages was established. We labeled Sox9(+) mouse liver cells at low density with a multicolor fluorescent confetti reporter. Organoid formation validated the progenitor activity of the labeled population. Sox9(+) cells were traced in multiple oval cell injury models using both histology and fluorescence-activated cell sorting. Surprisingly, only rare clones containing both hepatocytes and oval cells were found in any experiment. Quantitative analysis showed that Sox9(+) cells contributed only minimally (<1%) to the hepatocyte pool, even in classic oval cell injury models. In contrast, clonally marked mature hepatocytes demonstrated the ability to self-renew in all classic mouse oval cell activation injuries. A hepatocyte chimera model to trace hepatocytes and nonparenchymal cells also demonstrated the prevalence of hepatocyte-driven regeneration in mouse oval cell injury models.
Conclusion: Sox9(+) ductal progenitor cells give rise to clonal oval cell proliferation and bipotential organoids, but rarely produce hepatocytes in vivo. Hepatocytes themselves are the predominant source of new parenchyma cells in prototypical mouse models of oval cell activation.
© 2014 by the American Association for the Study of Liver Diseases.
Figures




References
-
- Farber E. Similarities in the sequence of early histological changes induced in the liver of the rat by ethionine, 2-acetylamino-fluorene, and 3’-methyl-4-dimethylaminoazobenzene. Cancer Res. 1956;16:142–148. - PubMed
-
- Sell S. Comparison of liver progenitor cells in human atypical ductular reactions with those seen in experimental models of liver injury. Hepatology. 1998;27:317–331. - PubMed
-
- Roskams T, De Vos R, Van Eyken P, Myazaki H, Van Damme B, Desmet V. Hepatic OV-6 expression in human liver disease and rat experiments: evidence for hepatic progenitor cells in man. Journal of Hepatology. 1998;29:455–463. - PubMed
-
- Itoh T, Miyajima A. Liver regeneration by stem/progenitor cells. Hepatology. 2013 - PubMed
-
- Grisham JW, Porta E. Origin and fate of proliferated hepatic ductal cells in the rate: electron microscopic and autoradiographic studies. Exp Mol Pathol. 1964;86:242–261. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
