Intein applications: from protein purification and labeling to metabolic control methods
- PMID: 24700459
- PMCID: PMC4031509
- DOI: 10.1074/jbc.R114.552653
Intein applications: from protein purification and labeling to metabolic control methods
Abstract
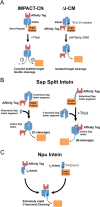
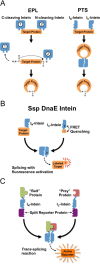
The discovery of inteins in the early 1990s opened the door to a wide variety of new technologies. Early engineered inteins from various sources allowed the development of self-cleaving affinity tags and new methods for joining protein segments through expressed protein ligation. Some applications were developed around native and engineered split inteins, which allow protein segments expressed separately to be spliced together in vitro. More recently, these early applications have been expanded and optimized through the discovery of highly efficient trans-splicing and trans-cleaving inteins. These new inteins have enabled a wide variety of applications in metabolic engineering, protein labeling, biomaterials construction, protein cyclization, and protein purification.
Keywords: Intein; Protein Chemical Modification; Protein Cyclization; Protein Engineering; Protein Labeling; Protein Processing; Protein Purification; Protein Self-assembly; Protein Splicing; Self-cleaving Tag.
© 2014 by The American Society for Biochemistry and Molecular Biology, Inc.
Figures
References
-
- Southworth M. W., Amaya K., Evans T. C., Xu M. Q., Perler F. B. (1999) Purification of proteins fused to either the amino or carboxy terminus of the Mycobacterium xenopi gyrase A intein. Biotechniques 27, 110–114, 116,, 118–120 - PubMed
-
- Chong S., Mersha F. B., Comb D. G., Scott M. E., Landry D., Vence L. M., Perler F. B., Benner J., Kucera R. B., Hirvonen C. A., Pelletier J. J., Paulus H., Xu M.-Q. (1997) Single-column purification of free recombinant proteins using a self-cleavable affinity tag derived from a protein splicing element. Gene 192, 271–281 - PubMed
-
- Szweda P., Pladzyk R., Kotlowski R., Kur J. (2001) Cloning, expression, and purification of the Staphylococcus simulans lysostaphin using the intein-chitin-binding domain (CBD) system. Protein Expr. Purif. 22, 467–471 - PubMed
-
- Hong S., Toyama M., Maret W., Murooka Y. (2001) High yield expression and single step purification of human thionein/metallothionein. Protein Expr. Purif. 21, 243–250 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

