The association of cortactin with profilin-1 is critical for smooth muscle contraction
- PMID: 24700464
- PMCID: PMC4022883
- DOI: 10.1074/jbc.M114.548099
The association of cortactin with profilin-1 is critical for smooth muscle contraction
Abstract
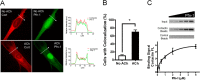
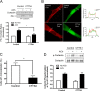
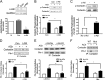
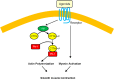
Profilin-1 (Pfn-1) is an actin-regulatory protein that has a role in modulating smooth muscle contraction. However, the mechanisms that regulate Pfn-1 in smooth muscle are not fully understood. Here, stimulation with acetylcholine induced an increase in the association of the adapter protein cortactin with Pfn-1 in smooth muscle cells/tissues. Furthermore, disruption of the protein/protein interaction by a cell-permeable peptide (CTTN-I peptide) attenuated actin polymerization and smooth muscle contraction without affecting myosin light chain phosphorylation at Ser-19. Knockdown of cortactin by lentivirus-mediated RNAi also diminished actin polymerization and smooth muscle force development. However, cortactin knockdown did not affect myosin activation. In addition, cortactin phosphorylation has been implicated in nonmuscle cell migration. In this study, acetylcholine stimulation induced cortactin phosphorylation at Tyr-421 in smooth muscle cells. Phenylalanine substitution at this position impaired cortactin/Pfn-1 interaction in response to contractile activation. c-Abl is a tyrosine kinase that is necessary for actin dynamics and contraction in smooth muscle. Here, c-Abl silencing inhibited the agonist-induced cortactin phosphorylation and the association of cortactin with Pfn-1. Finally, treatment with CTTN-I peptide reduced airway resistance and smooth muscle hyperreactivity in a murine model of asthma. These results suggest that the interaction of cortactin with Pfn-1 plays a pivotal role in regulating actin dynamics, smooth muscle contraction, and airway hyperresponsiveness in asthma. The association of cortactin with Pfn-1 is regulated by c-Abl-mediated cortactin phosphorylation.
Keywords: Actin; Adapter Protein; Contraction; Cytoskeleton; Excitation-Contraction Coupling; Protein Phosphorylation; Smooth Muscle.
© 2014 by The American Society for Biochemistry and Molecular Biology, Inc.
Figures






Similar articles
-
Role and regulation of Abelson tyrosine kinase in Crk-associated substrate/profilin-1 interaction and airway smooth muscle contraction.Respir Res. 2018 Jan 5;19(1):4. doi: 10.1186/s12931-017-0709-4. Respir Res. 2018. PMID: 29304860 Free PMC article.
-
Role of c-Abl tyrosine kinase in smooth muscle cell migration.Am J Physiol Cell Physiol. 2014 Apr 15;306(8):C753-61. doi: 10.1152/ajpcell.00327.2013. Epub 2014 Jan 29. Am J Physiol Cell Physiol. 2014. PMID: 24477238 Free PMC article.
-
Role of the adapter protein Abi1 in actin-associated signaling and smooth muscle contraction.J Biol Chem. 2013 Jul 12;288(28):20713-22. doi: 10.1074/jbc.M112.439877. Epub 2013 Jun 5. J Biol Chem. 2013. PMID: 23740246 Free PMC article.
-
Critical role of actin-associated proteins in smooth muscle contraction, cell proliferation, airway hyperresponsiveness and airway remodeling.Respir Res. 2015 Oct 30;16:134. doi: 10.1186/s12931-015-0296-1. Respir Res. 2015. PMID: 26517982 Free PMC article. Review.
-
The Dynamic Actin Cytoskeleton in Smooth Muscle.Adv Pharmacol. 2018;81:1-38. doi: 10.1016/bs.apha.2017.06.001. Epub 2017 Aug 24. Adv Pharmacol. 2018. PMID: 29310796 Review.
Cited by
-
Distinctive roles of Abi1 in regulating actin-associated proteins during human smooth muscle cell migration.Sci Rep. 2020 Jun 30;10(1):10667. doi: 10.1038/s41598-020-67781-1. Sci Rep. 2020. PMID: 32606387 Free PMC article.
-
Plk1 Mediates Paxillin Phosphorylation (Ser-272), Centrosome Maturation, and Airway Smooth Muscle Layer Thickening in Allergic Asthma.Sci Rep. 2019 May 17;9(1):7555. doi: 10.1038/s41598-019-43927-8. Sci Rep. 2019. PMID: 31101859 Free PMC article.
-
Smooth Muscle Myosin Localizes at the Leading Edge and Regulates the Redistribution of Actin-regulatory Proteins during Migration.Cells. 2022 Jul 29;11(15):2334. doi: 10.3390/cells11152334. Cells. 2022. PMID: 35954178 Free PMC article.
-
MicroRNA miR-509 Regulates ERK1/2, the Vimentin Network, and Focal Adhesions by Targeting Plk1.Sci Rep. 2018 Aug 22;8(1):12635. doi: 10.1038/s41598-018-30895-8. Sci Rep. 2018. PMID: 30135525 Free PMC article.
-
Pathobiology of Airway Remodeling in Asthma: The Emerging Role of Integrins.J Asthma Allergy. 2022 May 11;15:595-610. doi: 10.2147/JAA.S267222. eCollection 2022. J Asthma Allergy. 2022. PMID: 35592385 Free PMC article. Review.
References
-
- Kamm K. E., Stull J. T. (1989) Regulation of smooth muscle contractile elements by second messengers. Annu. Rev. Physiol. 51, 299–313 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

