Centrobin-centrosomal protein 4.1-associated protein (CPAP) interaction promotes CPAP localization to the centrioles during centriole duplication
- PMID: 24700465
- PMCID: PMC4140877
- DOI: 10.1074/jbc.M113.531152
Centrobin-centrosomal protein 4.1-associated protein (CPAP) interaction promotes CPAP localization to the centrioles during centriole duplication
Abstract
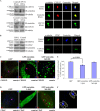
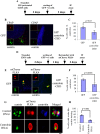
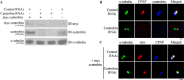
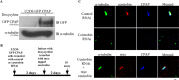
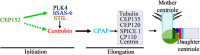
Centriole duplication is the process by which two new daughter centrioles are generated from the proximal end of preexisting mother centrioles. Accurate centriole duplication is important for many cellular and physiological events, including cell division and ciliogenesis. Centrosomal protein 4.1-associated protein (CPAP), centrosomal protein of 152 kDa (CEP152), and centrobin are known to be essential for centriole duplication. However, the precise mechanism by which they contribute to centriole duplication is not known. In this study, we show that centrobin interacts with CEP152 and CPAP, and the centrobin-CPAP interaction is critical for centriole duplication. Although depletion of centrobin from cells did not have an effect on the centriolar levels of CEP152, it caused the disappearance of CPAP from both the preexisting and newly formed centrioles. Moreover, exogenous expression of the CPAP-binding fragment of centrobin also caused the disappearance of CPAP from both the preexisting and newly synthesized centrioles, possibly in a dominant negative manner, thereby inhibiting centriole duplication and the PLK4 overexpression-mediated centrosome amplification. Interestingly, exogenous overexpression of CPAP in the centrobin-depleted cells did not restore CPAP localization to the centrioles. However, restoration of centrobin expression in the centrobin-depleted cells led to the reappearance of centriolar CPAP. Hence, we conclude that centrobin-CPAP interaction is critical for the recruitment of CPAP to procentrioles to promote the elongation of daughter centrioles and for the persistence of CPAP on preexisting mother centrioles. Our study indicates that regulation of CPAP levels on the centrioles by centrobin is critical for preserving the normal size, shape, and number of centrioles in the cell.
Keywords: CEP152; CPAP; Centriole; Centrobin; Centrosome; Cloning; Confocal Microscopy; Gene Expression.
© 2014 by The American Society for Biochemistry and Molecular Biology, Inc.
Figures


Similar articles
-
Centrobin-tubulin interaction is required for centriole elongation and stability.J Cell Biol. 2011 May 16;193(4):711-25. doi: 10.1083/jcb.201006135. J Cell Biol. 2011. PMID: 21576394 Free PMC article.
-
Centrobin-mediated regulation of the centrosomal protein 4.1-associated protein (CPAP) level limits centriole length during elongation stage.J Biol Chem. 2015 Mar 13;290(11):6890-902. doi: 10.1074/jbc.M114.603423. Epub 2015 Jan 23. J Biol Chem. 2015. PMID: 25616662 Free PMC article.
-
CEP120 interacts with CPAP and positively regulates centriole elongation.J Cell Biol. 2013 Jul 22;202(2):211-9. doi: 10.1083/jcb.201212060. Epub 2013 Jul 15. J Cell Biol. 2013. PMID: 23857771 Free PMC article.
-
The PLK4-STIL-SAS-6 module at the core of centriole duplication.Biochem Soc Trans. 2016 Oct 15;44(5):1253-1263. doi: 10.1042/BST20160116. Biochem Soc Trans. 2016. PMID: 27911707 Free PMC article. Review.
-
The centrosome duplication cycle in health and disease.FEBS Lett. 2014 Aug 1;588(15):2366-72. doi: 10.1016/j.febslet.2014.06.030. Epub 2014 Jun 18. FEBS Lett. 2014. PMID: 24951839 Review.
Cited by
-
Centrobin serves as a safeguard to guide timely centriole maturation during the cell cycle.Sci Rep. 2025 Mar 18;15(1):9280. doi: 10.1038/s41598-025-94414-2. Sci Rep. 2025. PMID: 40102598 Free PMC article.
-
Regulation of the centrosome cycle.Mol Cell Oncol. 2015 Jul 29;3(2):e1075643. doi: 10.1080/23723556.2015.1075643. eCollection 2016 Mar. Mol Cell Oncol. 2015. PMID: 27308597 Free PMC article. Review.
-
Centrobin is essential for C-tubule assembly and flagellum development in Drosophila melanogaster spermatogenesis.J Cell Biol. 2018 Jul 2;217(7):2365-2372. doi: 10.1083/jcb.201801032. Epub 2018 Apr 30. J Cell Biol. 2018. PMID: 29712734 Free PMC article.
-
Interactions of N- and C-terminal parts of Ana1 permitting centriole duplication but not elongation.bioRxiv [Preprint]. 2024 Oct 31:2024.10.28.620588. doi: 10.1101/2024.10.28.620588. bioRxiv. 2024. Update in: Open Biol. 2025 Feb;15(2):240325. doi: 10.1098/rsob.240325. PMID: 39554154 Free PMC article. Updated. Preprint.
-
The SON RNA splicing factor is required for intracellular trafficking structures that promote centriole assembly and ciliogenesis.Mol Biol Cell. 2021 Oct 1;32(20):ar4. doi: 10.1091/mbc.E21-06-0305. Epub 2021 Aug 18. Mol Biol Cell. 2021. PMID: 34406792 Free PMC article.
References
-
- Lüders J., Stearns T. (2007) Microtubule-organizing centres: a re-evaluation. Nat. Rev. Mol. Cell Biol. 8, 161–167 - PubMed
-
- Bornens M. (2002) Centrosome composition and microtubule anchoring mechanisms. Curr. Opin. Cell Biol. 14, 25–34 - PubMed
-
- Job D., Valiron O., Oakley B. (2003) Microtubule nucleation. Curr. Opin. Cell Biol. 15, 111–117 - PubMed
-
- Nigg E. A., Raff J. W. (2009) Centrioles, centrosomes, and cilia in health and disease. Cell 139, 663–678 - PubMed
-
- Kitagawa D., Kohlmaier G., Keller D., Strnad P., Balestra F. R., Flückiger I., Gönczy P. (2011) Spindle positioning in human cells relies on proper centriole formation and on the microcephaly proteins CPAP and STIL. J. Cell Sci. 124, 3884–3893 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

