Critical role of aquaporins in interleukin 1β (IL-1β)-induced inflammation
- PMID: 24700466
- PMCID: PMC4022865
- DOI: 10.1074/jbc.M113.534594
Critical role of aquaporins in interleukin 1β (IL-1β)-induced inflammation
Abstract
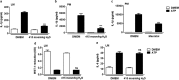
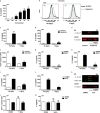
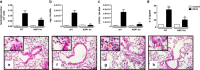
Rapid changes in cell volume characterize macrophage activation, but the role of water channels in inflammation remains unclear. We show here that, in vitro, aquaporin (AQP) blockade or deficiency results in reduced IL-1β release by macrophages activated with a variety of NLRP3 activators. Inhibition of AQP specifically during the regulatory volume decrease process is sufficient to limit IL-1β release by macrophages through the NLRP3 inflammasome axis. The immune-related activity of AQP was confirmed in vivo in a model of acute lung inflammation induced by crystals. AQP1 deficiency is associated with a marked reduction of both lung IL-1β release and neutrophilic inflammation. We conclude that AQP-mediated water transport in macrophages constitutes a general danger signal required for NLRP3-related inflammation. Our findings reveal a new function of AQP in the inflammatory process and suggest a novel therapeutic target for anti-inflammatory therapy.
Keywords: Aquaporin; Caspase; Cell Swelling; Inflammasome; Inflammation; Interleukin; RVD; Water Channel.
© 2014 by The American Society for Biochemistry and Molecular Biology, Inc.
Figures


Similar articles
-
Glucose regulates hypoxia-induced NLRP3 inflammasome activation in macrophages.J Cell Physiol. 2020 Oct;235(10):7554-7566. doi: 10.1002/jcp.29659. Epub 2020 Mar 1. J Cell Physiol. 2020. PMID: 32115713
-
Cyclooxygenase-2 regulates NLRP3 inflammasome-derived IL-1β production.J Cell Physiol. 2015 Apr;230(4):863-74. doi: 10.1002/jcp.24815. J Cell Physiol. 2015. PMID: 25294243
-
NLRP3 inflammasome-mediated neutrophil recruitment and hypernociception depend on leukotriene B(4) in a murine model of gout.Arthritis Rheum. 2012 Feb;64(2):474-84. doi: 10.1002/art.33355. Arthritis Rheum. 2012. PMID: 21952942
-
The anti-tumorigenic mushroom Agaricus blazei Murill enhances IL-1β production and activates the NLRP3 inflammasome in human macrophages.PLoS One. 2012;7(7):e41383. doi: 10.1371/journal.pone.0041383. Epub 2012 Jul 23. PLoS One. 2012. PMID: 22844468 Free PMC article.
-
The NLRP3 inflammasome in health and disease: the good, the bad and the ugly.Clin Exp Immunol. 2011 Oct;166(1):1-15. doi: 10.1111/j.1365-2249.2011.04440.x. Epub 2011 Jul 15. Clin Exp Immunol. 2011. PMID: 21762124 Free PMC article. Review.
Cited by
-
Pharmacological Improvement of Cystic Fibrosis Transmembrane Conductance Regulator Function Rescues Airway Epithelial Homeostasis and Host Defense in Children with Cystic Fibrosis.Am J Respir Crit Care Med. 2024 Jun 1;209(11):1338-1350. doi: 10.1164/rccm.202310-1836OC. Am J Respir Crit Care Med. 2024. PMID: 38259174 Free PMC article.
-
Aquaporin-1 attenuates macrophage-mediated inflammatory responses by inhibiting p38 mitogen-activated protein kinase activation in lipopolysaccharide-induced acute kidney injury.Inflamm Res. 2019 Dec;68(12):1035-1047. doi: 10.1007/s00011-019-01285-1. Epub 2019 Sep 16. Inflamm Res. 2019. PMID: 31529146 Free PMC article.
-
Distinct Pro-Inflammatory Mechanisms Elicited by Short and Long Amosite Asbestos Fibers in Macrophages.Int J Mol Sci. 2023 Oct 13;24(20):15145. doi: 10.3390/ijms242015145. Int J Mol Sci. 2023. PMID: 37894824 Free PMC article.
-
Aquaporins in sepsis- an update.Front Immunol. 2024 Oct 31;15:1495206. doi: 10.3389/fimmu.2024.1495206. eCollection 2024. Front Immunol. 2024. PMID: 39544938 Free PMC article. Review.
-
Stress-induced Neuroinflammation of the Spinal Cord is Restrained by Cort113176 (Dazucorilant), A Specific Glucocorticoid Receptor Modulator.Mol Neurobiol. 2024 Jan;61(1):1-14. doi: 10.1007/s12035-023-03554-x. Epub 2023 Aug 11. Mol Neurobiol. 2024. PMID: 37566177
References
-
- Thomas P. G., Dash P., Aldridge J. R., Jr., Ellebedy A. H., Reynolds C., Funk A. J., Martin W. J., Lamkanfi M., Webby R. J., Boyd K. L., Doherty P. C., Kanneganti T. D. (2009) The intracellular sensor NLRP3 mediates key innate and healing responses to influenza A virus via the regulation of caspase-1. Immunity 30, 566–575 - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

