Conjugated bile acids promote cholangiocarcinoma cell invasive growth through activation of sphingosine 1-phosphate receptor 2
- PMID: 24700501
- PMCID: PMC4141906
- DOI: 10.1002/hep.27085
Conjugated bile acids promote cholangiocarcinoma cell invasive growth through activation of sphingosine 1-phosphate receptor 2
Abstract
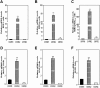
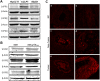
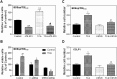
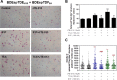
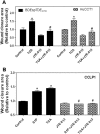
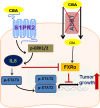
Cholangiocarcinoma (CCA) is an often fatal primary malignancy of the intra- and extrahepatic biliary tract that is commonly associated with chronic cholestasis and significantly elevated levels of primary and conjugated bile acids (CBAs), which are correlated with bile duct obstruction (BDO). BDO has also recently been shown to promote CCA progression. However, whereas there is increasing evidence linking chronic cholestasis and abnormal bile acid profiles to CCA development and progression, the specific mechanisms by which bile acids may be acting to promote cholangiocarcinogenesis and invasive biliary tumor growth have not been fully established. Recent studies have shown that CBAs, but not free bile acids, stimulate CCA cell growth, and that an imbalance in the ratio of free to CBAs may play an important role in the tumorigenesis of CCA. Also, CBAs are able to activate extracellular signal-regulated kinase (ERK)1/2- and phosphatidylinositol-3-kinase/protein kinase B (AKT)-signaling pathways through sphingosine 1-phosphate receptor 2 (S1PR2) in rodent hepatocytes. In the current study, we demonstrate S1PR2 to be highly expressed in rat and human CCA cells, as well as in human CCA tissues. We further show that CBAs activate the ERK1/2- and AKT-signaling pathways and significantly stimulate CCA cell growth and invasion in vitro. Taurocholate (TCA)-mediated CCA cell proliferation, migration, and invasion were significantly inhibited by JTE-013, a chemical antagonist of S1PR2, or by lentiviral short hairpin RNA silencing of S1PR2. In a novel organotypic rat CCA coculture model, TCA was further found to significantly increase the growth of CCA cell spheroidal/"duct-like" structures, which was blocked by treatment with JTE-013.
Conclusion: Our collective data support the hypothesis that CBAs promote CCA cell-invasive growth through S1PR2.
Copyright © 2014 The Authors. Hepatology published by Wiley on behalf of the American Association for the Study of Liver Diseases.
Figures


Comment in
-
Cholangiocarcinoma development: the resurgence of bile acids.Hepatology. 2014 Sep;60(3):795-7. doi: 10.1002/hep.27223. Epub 2014 Jul 17. Hepatology. 2014. PMID: 24828905 No abstract available.
References
-
- Sirica AE, Zhang Z, Lai GH, Asano T, Shen XN, Ward DJ, et al. A novel “patient-like” model of cholangiocarcinoma progression based on bile duct inoculation of tumorigenic rat cholangiocyte cell lines. Hepatology. 2008;47:1178–1190. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous
