Overlapping demyelinating syndromes and anti–N-methyl-D-aspartate receptor encephalitis
- PMID: 24700511
- PMCID: PMC4016175
- DOI: 10.1002/ana.24117
Overlapping demyelinating syndromes and anti–N-methyl-D-aspartate receptor encephalitis
Abstract
Objective: To report the clinical, radiological, and immunological association of demyelinating disorders with anti–Nmethyl- D-aspartate receptor (NMDAR) encephalitis.
Methods: Clinical and radiological analysis was done of a cohort of 691 patients with anti-NMDAR encephalitis. Determination of antibodies to NMDAR, aquaporin-4 (AQP4), and myelin oligodendrocyte glycoprotein (MOG) was performed using brain immunohistochemistry and cell-based assays.
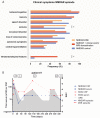
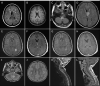
Results: Twenty-three of 691 patients with anti-NMDAR encephalitis had prominent magnetic resonance imaging (MRI) and/or clinical features of demyelination. Group 1 included 12 patients in whom anti-NMDAR encephalitis was preceded or followed by independent episodes of neuromyelitis optica (NMO) spectrum disorder (5 cases, 4 anti-AQP4 positive) or brainstem or multifocal demyelinating syndromes (7 cases, all anti-MOG positive). Group 2 included 11 patients in whom anti-NMDAR encephalitis occurred simultaneously with MRI and symptoms compatible with demyelination (5 AQ4 positive, 2 MOG positive). Group 3 (136 controls) included 50 randomly selected patients with typical anti-NMDAR encephalitis, 56 with NMO, and 30 with multiple sclerosis; NMDAR antibodies were detected only in the 50 anti-NMDAR patients, MOG antibodies in 3 of 50 anti-NMDAR and 1 of 56 NMO patients, and AQP4 antibodies in 48 of 56 NMO and 1 of 50 anti-NMDAR patients (p<0.0001 for all comparisons with Groups 1 and 2). Most patients improved with immunotherapy, but compared with anti-NMDAR encephalitis the demyelinating episodes required more intensive therapy and resulted in more residual deficits. Only 1 of 23 NMDAR patients with signs of demyelination had ovarian teratoma compared with 18 of 50 anti-NMDAR controls (p50.011).
Interpretation: Patients with anti-NMDAR encephalitis may develop concurrent or separate episodes of demyelinating disorders, and conversely patients with NMO or demyelinating disorders with atypical symptoms (eg, dyskinesias, psychosis) may have anti-NMDAR encephalitis.
Figures



Comment in
-
Reply: To PMID 24700511.Ann Neurol. 2014 Sep;76(3):464-5. doi: 10.1002/ana.24223. Epub 2014 Jul 26. Ann Neurol. 2014. PMID: 25043899 No abstract available.
-
Anti-N-methyl-D-aspartate receptor encephalitis with multiphasic demyelination.Ann Neurol. 2014 Sep;76(3):462-4. doi: 10.1002/ana.24224. Epub 2014 Jul 26. Ann Neurol. 2014. PMID: 25044002 No abstract available.
References
-
- Mikasova L, De RP, Bouchet D, et al. Disrupted surface cross-talk between NMDA and Ephrin-B2 receptors in anti-NMDA encephalitis. Brain. 2012;135:1606–1621. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

