Liver transplantation in the management of porphyria
- PMID: 24700519
- PMCID: PMC4498564
- DOI: 10.1002/hep.27086
Liver transplantation in the management of porphyria
Abstract
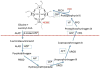
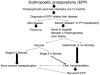
Porphyrias are a group of eight metabolic disorders, each resulting from a mutation that affects an enzyme of the heme biosynthetic pathway. Porphyrias are classified as hepatic or erythropoietic, depending upon the site where the gene defect is predominantly expressed. Clinical phenotypes are classified as follows: (1) acute porphyrias with neurovisceral symptoms: acute intermittent porphyria; delta amino-levulinic acid hydratase deficiency porphyria; hereditary coproporphyria; and variegate porphyria and (2) cutaneous porphyrias with skin blistering and photosensitivity: porphyria cutanea tarda; congenital erythropoietic porphyria; hepatoerythropoietic porphyria and both erythropoietic protoporphyrias: autosomal dominant and X-linked. Liver transplantation (LT) may be needed for recurrent and/or life-threatening acute attack in acute intermittent porphyria or acute liver failure or end-stage chronic liver disease in erythropoietic protoporphyria. LT in acute intermittent porphyria is curative. Erythropoietic protoporphyria patients needing LT should be considered for bone marrow transplantation to achieve cure.
Conclusion: This article provides an overview of porphyria with diagnostic approaches and management strategies for specific porphyrias and recommendations for LT with indications, pretransplant evaluation, and posttransplant management.
© 2014 by the American Association for the Study of Liver Diseases.
Conflict of interest statement
Potential conflict of interest: Nothing to report.
Figures

References
-
- Anderson KE, Bloomer JR, Bonkovsky HL, Kushner JP, Pierach CA, Pimstone NR, et al. Recommendations for the diagnosis and treatment of the acute porphyrias. Ann Intern Med. 2005;142:439–450. - PubMed
-
- Bonkovsky HL. Neurovisceral porphyrias: what a hematologist needs to know. Hematology Am Soc Hematol Educ Program. 2005:24–30. - PubMed
-
- Gouya L, Puy H, Lamoril J, Da Silva V, Grandchamp B, Nordmann Y, et al. Inheritance in erythropoietic protoporphyria: a common wildtype ferrochelatase allelic variant with low expression accounts for clinical manifestation. Blood. 1999;93:2105–2110. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
