Role of protein kinase C isoforms in bile formation and cholestasis
- PMID: 24700589
- PMCID: PMC4141907
- DOI: 10.1002/hep.27088
Role of protein kinase C isoforms in bile formation and cholestasis
Abstract
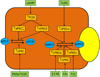
Transhepatic solute transport provides the osmotic driving force for canalicular bile formation. Choleretic and cholestatic agents affect bile formation, in part, by altering plasma membrane localizations of transporters involved in bile formation. These short-term dynamic changes in transporter location are highly regulated posttranslational events requiring various cellular signaling pathways. Interestingly, both choleretic and cholestatic agents activate the same intracellular signaling kinases, such as phosphoinositide-3-kinase (PI3K), protein kinase C (PKC), and mitogen-activated protein kinase (MAPK). An emerging theme is that choleretic and cholestatic effects may be mediated by different isoforms of these kinases. This is most evident for PKC-mediated regulation of plasma membrane localization of Na+-taurocholate cotransporting polypeptide (NTCP) and multidrug resistance-associated protein 2 (MRP2) by conventional PKCα (cPKCα), novel PKCδ (nPKCδ), nPKCε, and atypical PKCζ (aPKCζ). aPKCζ may mediate choleretic effects by inserting NTCP into the plasma membrane, and nPKCε may mediate cholestatic effects by retrieving MRP2 from the plasma membrane. On the other hand, cPKCα and nPKCδ may be involved in choleretic, cholestatic, and anticholestatic effects by inserting, retrieving, and inhibiting retrieval of transporters, respectively. The effects of PKC isoforms may be mediated by phosphorylation of the transporters, actin binding proteins (radixin and myristoylated alanine-rich C kinase substrate), and Rab proteins. Human NTCP plays an important role in the entry of hepatitis B and D viruses into hepatocytes and consequent infection. Thus, PKCs, by regulating NTCP trafficking, may also play an important role in hepatic viral infections.
© 2014 by the American Association for the Study of Liver Diseases.
Figures



Similar articles
-
Regulation of plasma membrane localization of the Na+-taurocholate cotransporting polypeptide (Ntcp) by hyperosmolarity and tauroursodeoxycholate.J Biol Chem. 2015 Oct 2;290(40):24237-54. doi: 10.1074/jbc.M115.666883. Epub 2015 Aug 25. J Biol Chem. 2015. PMID: 26306036 Free PMC article.
-
Protein kinase Cdelta mediates cyclic adenosine monophosphate-stimulated translocation of sodium taurocholate cotransporting polypeptide and multidrug resistant associated protein 2 in rat hepatocytes.Hepatology. 2008 Apr;47(4):1309-16. doi: 10.1002/hep.22162. Hepatology. 2008. PMID: 18273864
-
INTRACELLULAR SIGNALING BY BILE ACIDS.J Biosci (Rajshari). 2012;20:1-23. doi: 10.3329/jbs.v20i0.17647. J Biosci (Rajshari). 2012. PMID: 25378891 Free PMC article.
-
Sodium-dependent bile salt transporters of the SLC10A transporter family: more than solute transporters.Pflugers Arch. 2014 Jan;466(1):77-89. doi: 10.1007/s00424-013-1367-0. Epub 2013 Oct 3. Pflugers Arch. 2014. PMID: 24196564 Free PMC article. Review.
-
Hepatobiliary transport of bile acids and organic anions.J Hepatobiliary Pancreat Surg. 2002;9(4):443-7. doi: 10.1007/s005340200055. J Hepatobiliary Pancreat Surg. 2002. PMID: 12483266 Review.
Cited by
-
Melatonin ameliorates ANIT‑induced cholestasis by activating Nrf2 through a PI3K/Akt‑dependent pathway in rats.Mol Med Rep. 2019 Feb;19(2):1185-1193. doi: 10.3892/mmr.2018.9746. Epub 2018 Dec 12. Mol Med Rep. 2019. PMID: 30569102 Free PMC article.
-
Formulation of Liver-Specific PLGA-DY-635 Nanoparticles Loaded with the Protein Kinase C Inhibitor Bisindolylmaleimide I.Pharmaceutics. 2020 Nov 18;12(11):1110. doi: 10.3390/pharmaceutics12111110. Pharmaceutics. 2020. PMID: 33218172 Free PMC article.
-
The functional role of sodium taurocholate cotransporting polypeptide NTCP in the life cycle of hepatitis B, C and D viruses.Cell Mol Life Sci. 2018 Nov;75(21):3895-3905. doi: 10.1007/s00018-018-2892-y. Epub 2018 Aug 10. Cell Mol Life Sci. 2018. PMID: 30097692 Free PMC article. Review.
-
Mechanism of inhibition of taurolithocholate-induced retrieval of plasma membrane MRP2 by cyclic AMP and tauroursodeoxycholate.Physiol Rep. 2017 Dec;5(23):e13529. doi: 10.14814/phy2.13529. Physiol Rep. 2017. PMID: 29192063 Free PMC article.
-
Regulation of plasma membrane localization of the Na+-taurocholate cotransporting polypeptide (Ntcp) by hyperosmolarity and tauroursodeoxycholate.J Biol Chem. 2015 Oct 2;290(40):24237-54. doi: 10.1074/jbc.M115.666883. Epub 2015 Aug 25. J Biol Chem. 2015. PMID: 26306036 Free PMC article.
References
-
- Stapelbroek JM, van Erpecum KJ, Klomp LW, Houwen RH. Liver disease associated with canalicular transport defects: current and future therapies. J Hepatol. 2010;52:258–271. - PubMed
-
- Maillette de Buy WL, Beuers U. Bile salts and cholestasis. Dig Liver Dis. 2010;42:409–418. - PubMed
-
- Anwer MS. Cellular Regulation of hepatic bile acid transport in health and cholestasis. Hepatology. 2004;39:581–589. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
