Inorganic phosphate induces cancer cell mediated angiogenesis dependent on forkhead box protein C2 (FOXC2) regulated osteopontin expression
- PMID: 24700685
- PMCID: PMC4183733
- DOI: 10.1002/mc.22153
Inorganic phosphate induces cancer cell mediated angiogenesis dependent on forkhead box protein C2 (FOXC2) regulated osteopontin expression
Abstract
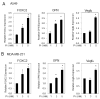
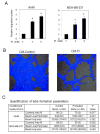
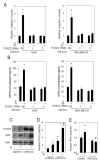
Recent studies in both rodents and humans suggest that elevated serum phosphorus, in the context of normal renal function, potentiates, or exacerbates pathologies associates with cardiovascular disease, bone metabolism, and cancer. Our recent microarray studies identified the potent stimulation of pro-angiogenic genes such as forkhead box protein C2 (FOXC2), osteopontin, and Vegfα, among others in response to elevated inorganic phosphate (Pi). Increased angiogenesis and neovascularization are important events in tumor growth and the progression to malignancy and FOXC2 has recently been identified as a potential transcriptional regulator of these processes. In this study we addressed the possibility that a high Pi environment would increase the angiogenic potential of cancer cells through a mechanism requiring FOXC2. Our studies utilized lung and breast cancer cell lines in combination with the human umbilical vascular endothelial cell (HUVEC) vessel formation model to better understand the mechanism(s) by which a high Pi environment might alter cancer progression. Exposure of cancer cells to elevated Pi stimulated expression of FOXC2 and conditioned medium from the Pi-stimulated cancer cells stimulated migration and tube formation in the HUVEC model. Mechanistically, we define the requirement of FOXC2 for Pi-induced osteopontin (OPN) expression and secretion from cancer cells as necessary for the angiogenic response. These studies reveal for the first time that cancer cells grown in a high Pi environment promote migration of endothelial cells and tube formation and in so doing identify a novel potential therapeutic target to reduce tumor progression.
Keywords: FOXC2; angiogenesis; inorganic phosphate; migration; osteopontin.
© 2014 Wiley Periodicals, Inc.
Conflict of interest statement
The authors have no conflicts of interest to declare.
Figures
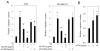
Similar articles
-
Endothelial immune activation programmes cell-fate decisions and angiogenesis by inducing angiogenesis regulator DLL4 through TLR4-ERK-FOXC2 signalling.J Physiol. 2018 Apr 15;596(8):1397-1417. doi: 10.1113/JP275453. Epub 2018 Mar 2. J Physiol. 2018. PMID: 29380370 Free PMC article.
-
Hypoxia-driven osteopontin contributes to breast tumor growth through modulation of HIF1α-mediated VEGF-dependent angiogenesis.Oncogene. 2014 Apr 17;33(16):2053-64. doi: 10.1038/onc.2013.171. Epub 2013 Jun 3. Oncogene. 2014. PMID: 23728336
-
Expression of transcription factor FOXC2 in cervical cancer and effects of silencing on cervical cancer cell proliferation.Asian Pac J Cancer Prev. 2014;15(4):1589-95. doi: 10.7314/apjcp.2014.15.4.1589. Asian Pac J Cancer Prev. 2014. PMID: 24641373
-
Foxc2 transcription factor: a newly described regulator of angiogenesis.Trends Cardiovasc Med. 2008 Aug;18(6):224-8. doi: 10.1016/j.tcm.2008.11.003. Trends Cardiovasc Med. 2008. PMID: 19185813 Free PMC article. Review.
-
Emerging roles and mechanisms of FOXC2 in cancer.Clin Chim Acta. 2018 Apr;479:84-93. doi: 10.1016/j.cca.2018.01.019. Epub 2018 Jan 16. Clin Chim Acta. 2018. PMID: 29341903 Review.
Cited by
-
Phosphate and Endothelial Function: How Sensing of Elevated Inorganic Phosphate Concentration Generates Signals in Endothelial Cells.Adv Exp Med Biol. 2022;1362:85-98. doi: 10.1007/978-3-030-91623-7_9. Adv Exp Med Biol. 2022. PMID: 35288875 Review.
-
Breast Cancer and Bone Mineral Density in a U.S. Cohort of Middle-Aged Women: Associations with Phosphate Toxicity.Cancers (Basel). 2023 Oct 21;15(20):5093. doi: 10.3390/cancers15205093. Cancers (Basel). 2023. PMID: 37894460 Free PMC article.
-
Excessive Inorganic Phosphate Burden Perturbed Intracellular Signaling: Quantitative Proteomics and Phosphoproteomics Analyses.Front Nutr. 2022 Jan 14;8:765391. doi: 10.3389/fnut.2021.765391. eCollection 2021. Front Nutr. 2022. PMID: 35096927 Free PMC article.
-
Dysregulated Phosphate Metabolism, Periodontal Disease, and Cancer: Possible Global Health Implications.Dent J (Basel). 2019 Feb 11;7(1):18. doi: 10.3390/dj7010018. Dent J (Basel). 2019. PMID: 30754693 Free PMC article.
-
Downregulation of PART1 Inhibits Proliferation and Differentiation of Hep3B Cells by Targeting hsa-miR-3529-3p/FOXC2 Axis.J Oncol. 2021 Aug 24;2021:7792223. doi: 10.1155/2021/7792223. eCollection 2021. J Oncol. 2021. PMID: 34484336 Free PMC article.
References
-
- Calvo MS. Dietary phosphorus, calcium metabolism and bone. The Journal of nutrition. 1993;123(9):1627–1633. - PubMed
-
- Kemi VE, Karkkainen MU, Karp HJ, Laitinen KA, Lamberg-Allardt CJ. Increased calcium intake does not completely counteract the effects of increased phosphorus intake on bone: an acute dose-response study in healthy females. The British journal of nutrition. 2008;99(4):832–839. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

