Rare bone diseases and their dental, oral, and craniofacial manifestations
- PMID: 24700690
- PMCID: PMC4107543
- DOI: 10.1177/0022034514529150
Rare bone diseases and their dental, oral, and craniofacial manifestations
Abstract
Hereditary diseases affecting the skeleton are heterogeneous in etiology and severity. Though many of these conditions are individually rare, the total number of people affected is great. These disorders often include dental-oral-craniofacial (DOC) manifestations, but the combination of the rarity and lack of in-depth reporting often limit our understanding and ability to diagnose and treat affected individuals. In this review, we focus on dental, oral, and craniofacial manifestations of rare bone diseases. Discussed are defects in 4 key physiologic processes in bone/tooth formation that serve as models for the understanding of other diseases in the skeleton and DOC complex: progenitor cell differentiation (fibrous dysplasia), extracellular matrix production (osteogenesis imperfecta), mineralization (familial tumoral calcinosis/hyperostosis hyperphosphatemia syndrome, hypophosphatemic rickets, and hypophosphatasia), and bone resorption (Gorham-Stout disease). For each condition, we highlight causative mutations (when known), etiopathology in the skeleton and DOC complex, and treatments. By understanding how these 4 foci are subverted to cause disease, we aim to improve the identification of genetic, molecular, and/or biologic causes, diagnoses, and treatment of these and other rare bone conditions that may share underlying mechanisms of disease.
Keywords: Gorham-Stout disease; familial hypophosphatemic rickets; fibrous dysplasia of bone; hyperphosphatemic familial tumoral calcinosis; hypophosphatasia; osteogenesis imperfecta.
© International & American Associations for Dental Research.
Conflict of interest statement
The authors declare no potential conflicts of interest with respect to the authorship and/or publication of this article.
Figures


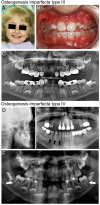

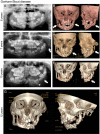
Similar articles
-
Rare diseases: a challenge in paediatric dentistry.Eur J Paediatr Dent. 2024 Sep 3;25(3):171-171. doi: 10.23804/ejpd.2024.25.03.01. Epub 2024 Sep 1. Eur J Paediatr Dent. 2024. PMID: 39212455 Review.
-
Genetic disorders and bone affecting the craniofacial skeleton.Oral Maxillofac Surg Clin North Am. 2007 Nov;19(4):467-74, v. doi: 10.1016/j.coms.2007.08.001. Oral Maxillofac Surg Clin North Am. 2007. PMID: 18088898 Review.
-
Metabolic Bone Disease: An Overview.Mo Med. 2024 Jul-Aug;121(4):297-303. Mo Med. 2024. PMID: 39575071 Free PMC article. Review.
-
Recessive mutation in GALNT3 causes hyperphosphatemic familial tumoral calcinosis associated with chronic recurrent multifocal osteomyelitis.Turk J Pediatr. 2019;61(1):130-133. doi: 10.24953/turkjped.2019.01.022. Turk J Pediatr. 2019. PMID: 31559735
-
Hyperphosphatemic familial tumoral calcinosis secondary to fibroblast growth factor 23 (FGF23) mutation: a report of two affected families and review of the literature.Osteoporos Int. 2018 Sep;29(9):1987-2009. doi: 10.1007/s00198-018-4574-x. Epub 2018 Jun 20. Osteoporos Int. 2018. PMID: 29923062 Review.
Cited by
-
Mandibular Gorham-Stout disease: A case report and literature review.Medicine (Baltimore). 2017 Oct;96(42):e8184. doi: 10.1097/MD.0000000000008184. Medicine (Baltimore). 2017. PMID: 29049202 Free PMC article. Review.
-
Dental Manifestations of Pediatric Bone Disorders.Curr Osteoporos Rep. 2017 Dec;15(6):588-592. doi: 10.1007/s11914-017-0409-5. Curr Osteoporos Rep. 2017. PMID: 28965204 Review.
-
Gene Therapy Using Adeno-Associated Virus Serotype 8 Encoding TNAP-D10 Improves the Skeletal and Dentoalveolar Phenotypes in Alpl-/- Mice.J Bone Miner Res. 2021 Sep;36(9):1835-1849. doi: 10.1002/jbmr.4382. Epub 2021 Jun 15. J Bone Miner Res. 2021. PMID: 34076297 Free PMC article.
-
Periodontal Defects in the A116T Knock-in Murine Model of Odontohypophosphatasia.J Dent Res. 2015 May;94(5):706-14. doi: 10.1177/0022034515573273. Epub 2015 Feb 25. J Dent Res. 2015. PMID: 25716980 Free PMC article.
-
Dentoalveolar Defects in the Hyp Mouse Model of X-linked Hypophosphatemia.J Dent Res. 2020 Apr;99(4):419-428. doi: 10.1177/0022034520901719. Epub 2020 Jan 24. J Dent Res. 2020. PMID: 31977267 Free PMC article.
References
-
- Akintoye SO, Lee JS, Feimster T, Booher S, Brahim J, Kingman A, et al. (2003). Dental characteristics of fibrous dysplasia and McCune-Albright syndrome. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 96:275-282. - PubMed
-
- Al-Jamali J, Glaum R, Kassem A, Voss PJ, Schmelzeisen R, Schon R. (2012). Gorham-Stout syndrome of the facial bones: a review of pathogenesis and treatment modalities and report of a case with a rare cutaneous manifestations [sic]. Oral Surg Oral Med Oral Pathol Oral Radiol 114:e23-e29. - PubMed
-
- Benet-Pages A, Orlik P, Strom TM, Lorenz-Depiereux B. (2005). An FGF23 missense mutation causes familial tumoral calcinosis with hyperphosphatemia. Hum Mol Genet 14:385-390. - PubMed
-
- Berdal A, Molla M, Descroix V. (2011). Vitamin D and oral health. In: Vitamin D. Feldman D, Pike JW, Adams JS, editors. San Diego, CA: Academic Press, pp. 521-532.
Publication types
MeSH terms
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

