Role in tumor growth of a glycogen debranching enzyme lost in glycogen storage disease
- PMID: 24700805
- PMCID: PMC4580555
- DOI: 10.1093/jnci/dju062
Role in tumor growth of a glycogen debranching enzyme lost in glycogen storage disease
Abstract
Background: Bladder cancer is the most common malignancy of the urinary system, yet our molecular understanding of this disease is incomplete, hampering therapeutic advances.
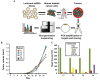
Methods: Here we used a genome-wide functional short-hairpin RNA (shRNA) screen to identify suppressors of in vivo bladder tumor xenograft growth (n = 50) using bladder cancer UMUC3 cells. Next-generation sequencing was used to identify the most frequently occurring shRNAs in tumors. Genes so identified were studied in 561 patients with bladder cancer for their association with stratification of clinical outcome by Kaplan-Meier analysis. The best prognostic marker was studied to determine its mechanism in tumor suppression using anchorage-dependent and -independent growth, xenograft (n = 20), and metabolomic assays. Statistical significance was determined using two-sided Student t test and repeated-measures statistical analysis.
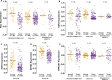
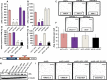
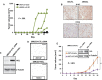
Results: We identified the glycogen debranching enzyme AGL as a prognostic indicator of patient survival (P = .04) and as a novel regulator of bladder cancer anchorage-dependent (P < .001), anchorage-independent (mean ± standard deviation, 180 ± 23.1 colonies vs 20±9.5 in control, P < .001), and xenograft growth (P < .001). Rescue experiments using catalytically dead AGL variants revealed that this effect is independent of AGL enzymatic functions. We demonstrated that reduced AGL enhances tumor growth by increasing glycine synthesis through increased expression of serine hydroxymethyltransferase 2.
Conclusions: Using an in vivo RNA interference screen, we discovered that AGL, a glycogen debranching enzyme, has a biologically and statistically significant role in suppressing human cancer growth.
© The Author 2014. Published by Oxford University Press. All rights reserved. For Permissions, please e-mail: journals.permissions@oup.com.
Figures



References
-
- Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin. 2013;63(1):11–30. - PubMed
-
- Gildea JJ, Golden WL, Harding MA, et al. Genetic and phenotypic changes associated with the acquisition of tumorigenicity in human bladder cancer. Genes Chromosomes Cancer. 2000;27(3):252–263. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

