Optical control of muscle function by transplantation of stem cell-derived motor neurons in mice
- PMID: 24700859
- PMCID: PMC5947756
- DOI: 10.1126/science.1248523
Optical control of muscle function by transplantation of stem cell-derived motor neurons in mice
Abstract
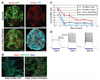
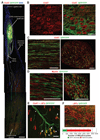
Damage to the central nervous system caused by traumatic injury or neurological disorders can lead to permanent loss of voluntary motor function and muscle paralysis. Here, we describe an approach that circumvents central motor circuit pathology to restore specific skeletal muscle function. We generated murine embryonic stem cell-derived motor neurons that express the light-sensitive ion channel channelrhodopsin-2, which we then engrafted into partially denervated branches of the sciatic nerve of adult mice. These engrafted motor neurons not only reinnervated lower hind-limb muscles but also enabled their function to be restored in a controllable manner using optogenetic stimulation. This synthesis of regenerative medicine and optogenetics may be a successful strategy to restore muscle function after traumatic injury or disease.
Figures

Comment in
-
Neuroscience. Optogenetic regeneration.Science. 2014 Apr 4;344(6179):44-5. doi: 10.1126/science.1253088. Science. 2014. PMID: 24700845 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

