AKI after conditional and kidney-specific knockdown of stanniocalcin-1
- PMID: 24700878
- PMCID: PMC4178431
- DOI: 10.1681/ASN.2013070690
AKI after conditional and kidney-specific knockdown of stanniocalcin-1
Abstract
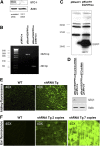
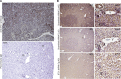
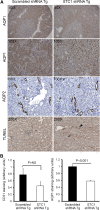
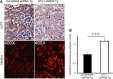
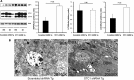
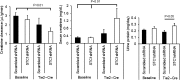
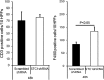
Stanniocalcin-1 is an intracrine protein; it binds to the cell surface, is internalized to the mitochondria, and diminishes superoxide generation through induction of uncoupling proteins. In vitro, stanniocalcin-1 inhibits macrophages and preserves endothelial barrier function, and transgenic overexpression of stanniocalcin-1 in mice protects against ischemia-reperfusion kidney injury. We sought to determine the kidney phenotype after kidney endothelium-specific expression of stanniocalcin-1 small hairpin RNA (shRNA). We generated transgenic mice that express stanniocalcin-1 shRNA or scrambled shRNA upon removal of a floxed reporter (phosphoglycerate kinase-driven enhanced green fluorescent protein) and used ultrasound microbubbles to deliver tyrosine kinase receptor-2 promoter-driven Cre to the kidney to permit kidney endothelium-specific shRNA expression. Stanniocalcin-1 mRNA and protein were expressed throughout the kidney in wild-type mice. Delivery of tyrosine kinase receptor-2 promoter-driven Cre to stanniocalcin-1 shRNA transgenic kidneys diminished the expression of stanniocalcin-1 mRNA and protein throughout the kidneys. Stanniocalcin-1 mRNA and protein expression did not change in similarly treated scrambled shRNA transgenic kidneys, and we observed no Cre protein expression in cultured and tyrosine kinase receptor-2 promoter-driven Cre-transfected proximal tubule cells, suggesting that knockdown of stanniocalcin-1 in epithelial cells in vivo may result from stanniocalcin-1 shRNA transfer from endothelial cells to epithelial cells. Kidney-specific knockdown of stanniocalcin-1 led to severe proximal tubule injury characterized by vacuolization, decreased uncoupling of protein-2 expression, greater generation of superoxide, activation of the unfolded protein response, initiation of autophagy, cell apoptosis, and kidney failure. Our observations suggest that stanniocalcin-1 is critical for tubular epithelial survival under physiologic conditions.
Copyright © 2014 by the American Society of Nephrology.
Figures




References
-
- De Niu P, Radman DP, Jaworski EM, Deol H, Gentz R, Su J, Olsen HS, Wagner GF: Development of a human stanniocalcin radioimmunoassay: Serum and tissue hormone levels and pharmacokinetics in the rat. Mol Cell Endocrinol 162: 131–144, 2000 - PubMed
-
- Luo CW, Kawamura K, Klein C, Hsueh AJ: Paracrine regulation of ovarian granulosa cell differentiation by stanniocalcin (STC) 1: Mediation through specific STC1 receptors. Mol Endocrinol 18: 2085–2096, 2004 - PubMed
-
- McCudden CR, James KA, Hasilo C, Wagner GF: Characterization of mammalian stanniocalcin receptors. Mitochondrial targeting of ligand and receptor for regulation of cellular metabolism. J Biol Chem 277: 45249–45258, 2002 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

