Efficacy and tolerability of renzapride in irritable bowel syndrome: a meta-analysis of randomized, controlled clinical trials including 2528 patients
- PMID: 24701208
- PMCID: PMC3953973
- DOI: 10.5114/aoms.2014.40729
Efficacy and tolerability of renzapride in irritable bowel syndrome: a meta-analysis of randomized, controlled clinical trials including 2528 patients
Abstract
Introduction: By targeting different subtypes of 5-hydroxytryptamine (5HT) receptors in the gastrointestinal (GI) tract, several drugs have been introduced for the management of irritable bowel syndrome (IBS). Renzapride is a full agonist for 5HT4 receptor and an antagonist to 5HT2b and 5HT3 receptors which is thought a promising therapeutic agent for constipation predominant IBS (C-IBS) patients due to its accelerating effect on the GI tract. In this meta-analysis, our aim was to evaluate the efficacy and tolerability of renzapride in the management of IBS.
Material and methods: A search was done from 1992 to February 2013 for placebo-controlled trials that investigated the efficacy of renzapride in IBS.
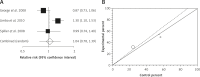
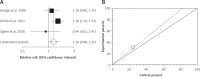
Results: Relative risk (RR) for clinical efficacy in IBS patients treated for 5 weeks or less comparing renzapride to placebo was 1.07 (95% CI = 0.89-1.29, p = 0.38). This value for IBS patients treated for more than 5 weeks was 1.04 (95% CI = 0.78-1.239, p = 0.77). The RR for clinical efficacy in IBS patients treated with renzapride (4 mg) for 5 weeks or less and more than 5 weeks in comparison to placebo was 1.2 (95% CI = 0.97-1.48, p = 0.1) and 1.16 (95% CI = 0.98-1.37, p = 0.08), respectively, which were statistically non-significant but clinically important. The analysis of tolerability demonstrated that amongst different reported adverse effects, renzapride caused diarrhea more than placebo (RR = 1.61 with a 95% CI = 1.16-2.24, p = 0.004). The RR for withdrawals from renzapride compared to placebo was 1.58 (95% CI = 1.26-2.07, p = 0.0007).
Conclusions: Renzapride is not superior to placebo in relieving IBS symptoms and causes significant incidences of diarrhea and drop-outs due to adverse effects in treated patients vs. placebo. Thus, this medicine might be a cost burden to patients without providing good effectiveness.
Keywords: 5-hydroxytryptamine; clinical trial; irritable bowel syndrome; meta-analysis; renzapride; systematic review.
Figures



Similar articles
-
Efficacy and tolerability of alosetron for the treatment of irritable bowel syndrome in women and men: a meta-analysis of eight randomized, placebo-controlled, 12-week trials.Clin Ther. 2008 May;30(5):884-901. doi: 10.1016/j.clinthera.2008.05.002. Clin Ther. 2008. PMID: 18555935
-
Clinical trial: renzapride treatment of women with irritable bowel syndrome and constipation - a double-blind, randomized, placebo-controlled, study.Aliment Pharmacol Ther. 2010 May;31(9):979-90. doi: 10.1111/j.1365-2036.2010.04265.x. Epub 2010 Feb 16. Aliment Pharmacol Ther. 2010. PMID: 20163375 Clinical Trial.
-
Effect of renzapride on transit in constipation-predominant irritable bowel syndrome.Clin Gastroenterol Hepatol. 2004 Oct;2(10):895-904. doi: 10.1016/s1542-3565(04)00391-x. Clin Gastroenterol Hepatol. 2004. PMID: 15476153 Clinical Trial.
-
Effects of linaclotide in patients with irritable bowel syndrome with constipation or chronic constipation: a meta-analysis.Clin Gastroenterol Hepatol. 2013 Sep;11(9):1084-1092.e3; quiz e68. doi: 10.1016/j.cgh.2013.04.032. Epub 2013 May 2. Clin Gastroenterol Hepatol. 2013. PMID: 23644388
-
Efficacy and Tolerability of Guanylate Cyclase-C Agonists for Irritable Bowel Syndrome with Constipation and Chronic Idiopathic Constipation: A Systematic Review and Meta-Analysis.Am J Gastroenterol. 2018 Mar;113(3):329-338. doi: 10.1038/ajg.2017.495. Epub 2018 Jan 30. Am J Gastroenterol. 2018. PMID: 29380823 Free PMC article.
Cited by
-
New and Investigational Agents for Irritable Bowel Syndrome.Curr Gastroenterol Rep. 2015 Dec;17(12):46. doi: 10.1007/s11894-015-0473-x. Curr Gastroenterol Rep. 2015. PMID: 26446557 Review.
-
New therapeutic perspectives in irritable bowel syndrome: Targeting low-grade inflammation, immuno-neuroendocrine axis, motility, secretion and beyond.World J Gastroenterol. 2017 Sep 28;23(36):6593-6627. doi: 10.3748/wjg.v23.i36.6593. World J Gastroenterol. 2017. PMID: 29085207 Free PMC article. Review.
-
Evidence-Based Clinical Guidelines for Chronic Constipation 2023.Digestion. 2025;106(1):62-89. doi: 10.1159/000540912. Epub 2024 Aug 19. Digestion. 2025. PMID: 39159626 Free PMC article.
-
Irritable Bowel Syndrome: Clinical Manifestations, Dietary Influences, and Management.Healthcare (Basel). 2017 Apr 26;5(2):21. doi: 10.3390/healthcare5020021. Healthcare (Basel). 2017. PMID: 28445436 Free PMC article. Review.
-
Clinical efficacy and safety of colistin treatment in patients with pulmonary infection caused by Pseudomonas aeruginosa or Acinetobacter baumannii: a meta-analysis.Arch Med Sci. 2015 Mar 16;11(1):34-42. doi: 10.5114/aoms.2015.48158. Epub 2015 Jan 8. Arch Med Sci. 2015. PMID: 25861288 Free PMC article. Review.
References
-
- Rahimi R, Nikfar S, Abdollahi M. Selective serotonin reuptake inhibitors for the management of irritable bowel syndrome: a meta-analysis of randomized controlled trials. Arch Med Sci. 2008;4:71–6.
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
