Local CTLA4 blockade effectively restrains experimental pancreatic adenocarcinoma growth in vivo
- PMID: 24701377
- PMCID: PMC3962508
- DOI: 10.4161/onci.27614
Local CTLA4 blockade effectively restrains experimental pancreatic adenocarcinoma growth in vivo
Abstract
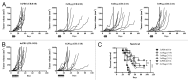
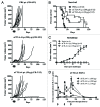
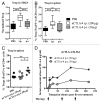
Antibody-mediated blockade of CTLA4 has been shown to be effective in treating a select group of patients with late-stage melanoma. The precise mechanism underlying the clinical activity of CTLA4 immunotherapy is poorly understood, although recent experimental findings indicate that antibody-mediated depletion of regulatory T cells (Tregs) in the tumor microenvironment plays a key role in efficacious antitumor responses. In the current study, we used an experimental model of pancreatic adenocarcinoma to compare the antitumor efficacy of peritumoral low-dose anti-CTLA4 monoclonal antibody (mAb) administration to that of a commonly utilized systemic high-dose anti-CTLA4 regimen. We selected pancreatic adenocarcinoma as it presents a particular challenge to clinicians due to its aggressive behavior, metastatic spread and limited treatment options. Furthermore, Fc gamma receptor (FcγR)-dense myeloid cells commonly infiltrate pancreatic tumors, such that these tumor types exhibit increased susceptibility to CTLA4 antibody-targeted Treg depletion via antibody-dependent cell-mediated cytotoxicity (ADCC). Locally administered anti-CTLA4 mAb effectively reduced tumor growth at a low dose and no additional anti-tumor effects were apparent when increasing the dose or number of injections. No significant difference in overall survival was seen when comparing locally administered low-dose with standard systemic high-dose CTLA4 blockade therapy, and both delivery routes led to increased tumor-infiltrating effector T cells and reduced Treg cells. As opposed to low-dose peritumoral treatment, high-dose systemic therapy stimulated the accumulation of Tregs in secondary lymphoid organs, an effect that could potentially counteract the antitumor immunotherapeutic benefit of CTLA4 blockade. Our study confirms previous findings that local administration of low-dose anti-CTLA4 antibody generates sustained antitumor effects and provides rationale to devise ultrasound-guided intratumoral anti-CTLA4 antibody injection regimens to treat patients with pancreatic adenocarcinoma and other types of solid tumors. In support, clinical relevancy could include reduced immune-related adverse events by limiting systemic antibody spread to immune cell-dense organs.
Keywords: anti-CTLA4; checkpoint blockade; immunotherapy; local therapy; pancreatic cancer.
Figures

Similar articles
-
Tumor-conditional anti-CTLA4 uncouples antitumor efficacy from immunotherapy-related toxicity.J Clin Invest. 2019 Jan 2;129(1):349-363. doi: 10.1172/JCI123391. Epub 2018 Dec 10. J Clin Invest. 2019. PMID: 30530991 Free PMC article.
-
Co-administration of RANKL and CTLA4 Antibodies Enhances Lymphocyte-Mediated Antitumor Immunity in Mice.Clin Cancer Res. 2017 Oct 1;23(19):5789-5801. doi: 10.1158/1078-0432.CCR-17-0606. Epub 2017 Jun 20. Clin Cancer Res. 2017. PMID: 28634284
-
Oncolytic Newcastle disease virus expressing a checkpoint inhibitor as a radioenhancing agent for murine melanoma.EBioMedicine. 2019 Nov;49:96-105. doi: 10.1016/j.ebiom.2019.10.032. Epub 2019 Oct 29. EBioMedicine. 2019. PMID: 31676387 Free PMC article.
-
The Next Immune-Checkpoint Inhibitors: PD-1/PD-L1 Blockade in Melanoma.Clin Ther. 2015 Apr 1;37(4):764-82. doi: 10.1016/j.clinthera.2015.02.018. Epub 2015 Mar 29. Clin Ther. 2015. PMID: 25823918 Free PMC article. Review.
-
[Progress of study on antitumor effects of antibody dependent cell mediated cytotoxicity--review].Zhongguo Shi Yan Xue Ye Xue Za Zhi. 2010 Oct;18(5):1370-5. Zhongguo Shi Yan Xue Ye Xue Za Zhi. 2010. PMID: 21129296 Review. Chinese.
Cited by
-
Tumor-directed immunotherapy can generate tumor-specific T cell responses through localized co-stimulation.Cancer Immunol Immunother. 2017 Jan;66(1):1-7. doi: 10.1007/s00262-016-1909-3. Epub 2016 Oct 6. Cancer Immunol Immunother. 2017. PMID: 27714433 Free PMC article. Review.
-
CTLA-4 in Regulatory T Cells for Cancer Immunotherapy.Cancers (Basel). 2021 Mar 22;13(6):1440. doi: 10.3390/cancers13061440. Cancers (Basel). 2021. PMID: 33809974 Free PMC article. Review.
-
Cancer DNA vaccines: current preclinical and clinical developments and future perspectives.J Exp Clin Cancer Res. 2019 Apr 5;38(1):146. doi: 10.1186/s13046-019-1154-7. J Exp Clin Cancer Res. 2019. PMID: 30953535 Free PMC article. Review.
-
Differential potency of regulatory T cell-mediated immunosuppression in kidney tumors compared to subcutaneous tumors.Oncoimmunology. 2014 Dec 21;3(11):e963395. doi: 10.4161/21624011.2014.963395. eCollection 2014 Nov. Oncoimmunology. 2014. PMID: 25941590 Free PMC article.
-
CAR T-cell therapy for pancreatic cancer.J Surg Oncol. 2017 Jul;116(1):63-74. doi: 10.1002/jso.24627. Epub 2017 Mar 27. J Surg Oncol. 2017. PMID: 28346697 Free PMC article. Review.
References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
