Clinical experience with Liraglutide in 196 patients with type 2 diabetes from a tertiary care center in India
- PMID: 24701434
- PMCID: PMC3968738
- DOI: 10.4103/2230-8210.126572
Clinical experience with Liraglutide in 196 patients with type 2 diabetes from a tertiary care center in India
Abstract
Context: GLP-1 receptor agonists (GLP-1 RA) are unique antidiabetic agents that have the ability to lower blood glucose without causing hypoglycemia, while at the same time promoting weight loss. Information on the efficacy and safety of GLP-1 RA in the Indian diabetic population is limited.
Aims: (1) To evaluate the effect of GLP-1 RA, Liraglutide on glycemic control, and weight in obese Indian patients with type 2 diabetes. (2) To study the adverse event profile of Liraglutide in these patients in real-world clinical setting.
Settings and design: Observational study conducted in a tertiary care hospital.
Materials and methods: Liraglutide was prescribed to 196 obese patients with type 2 diabetes who had poor glycemic control on oral medications ± insulin. The initial dose of Liraglutide was 0.6 mg, which was up-titrated to 1.2 mg after 1 week; further up-titration to 1.8 mg was done based on tolerance. Dipeptidyl peptidase-IV (DPP-IV) inhibitors were discontinued and dose of other medications adjusted according to clinical judgment during the study period.
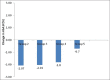
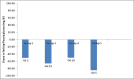
Results: Mean age of patients was 49.9 ± 9.6 years. Three month data were available for 175 patients out of a total of 196. At 3 months, glycosylated hemoglobin (HbA1c) was 7.6 ± 0.9% vs. 9.2 ± 1.9% at baseline (P = 0.007) and mean body weight was 96.0 ± 16.5 kg vs. 100.1 ± 17.5 kg at baseline (P < 0.001). Most common adverse events were nausea, burping, and eructation (10%).
Conclusion: Liraglutide significantly improves glycemic control with low risk of hypoglycemia and is associated with significant weight loss in obese Indian patients with type 2 diabetes mellitus.
Keywords: GLP-1 receptor agonists; India; obesity; type 2 diabetes.
Conflict of interest statement
Figures


References
-
- Anjana RM, Pradeepa R, Deepa M, Datta M, Sudha V, Unnikrishnan R, et al. ICMR-INDIAB Collaborative Study Group. Prevalence of diabetes and prediabetes (impaired fasting glucose and/or impaired glucose tolerance) in urban and rural India: Phase I results of the Indian Council of Medical Research-INdia DIABetes (ICMR-INDIAB) study. Diabetologia. 2011;54:3022–7. - PubMed
-
- Hoerger TJ, Segel JE, Gregg EW, Saaddine JB. Is glycemic control improving in U.S. adults? Diabetes Care. 2008;31:81–6. - PubMed
-
- Harris SB, Ekoe JM, Zdanowicz Y, Webster-Bogaert S. Glycemic control and morbidity in the Canadian primary care setting (results of the diabetes in Canada evaluation study) Diabetes Res Clin Pract. 2005;70:90–7. - PubMed
-
- Uwaifo GI, Ratner RE. Novel pharmacologic agents for type 2 diabetes. Endocrinol Metab Clin North Am. 2005;34:155–97. - PubMed
-
- Gonzalez C, Beruto V, Keller G, Santoro S, Di Girolamo G. Investigational treatments for Type 2 diabetes mellitus: Exenatide and liraglutide. Expert Opin Investig Drugs. 2006;15:887–95. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

