Bacterial multidrug efflux transporters
- PMID: 24702006
- PMCID: PMC4769028
- DOI: 10.1146/annurev-biophys-051013-022855
Bacterial multidrug efflux transporters
Abstract
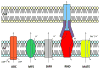
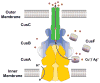
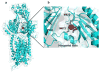
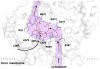
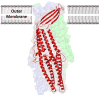
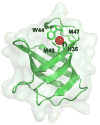
Infections caused by bacteria are a leading cause of death worldwide. Although antibiotics remain a key clinical therapy, their effectiveness has been severely compromised by the development of drug resistance in bacterial pathogens. Multidrug efflux transporters--a common and powerful resistance mechanism--are capable of extruding a number of structurally unrelated antimicrobials from the bacterial cell, including antibiotics and toxic heavy metal ions, facilitating their survival in noxious environments. Transporters of the resistance-nodulation-cell division (RND) superfamily typically assemble as tripartite efflux complexes spanning the inner and outer membranes of the cell envelope. In Escherichia coli, the CusCFBA complex, which mediates resistance to copper(I) and silver(I) ions, is the only known RND transporter specific to heavy metals. Here, we describe the current knowledge of individual pump components of the Cus system, a paradigm for efflux machinery, and speculate on how RND pumps assemble to fight diverse antimicrobials.
Keywords: CusCFBA efflux system; heavy metal resistance; multidrug resistance; resistance-nodulation-cell division.
Figures


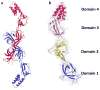


Similar articles
-
Heavy metal transport by the CusCFBA efflux system.Protein Sci. 2015 Nov;24(11):1720-36. doi: 10.1002/pro.2764. Epub 2015 Aug 24. Protein Sci. 2015. PMID: 26258953 Free PMC article. Review.
-
Structural mechanisms of heavy-metal extrusion by the Cus efflux system.Biometals. 2013 Aug;26(4):593-607. doi: 10.1007/s10534-013-9628-0. Epub 2013 May 9. Biometals. 2013. PMID: 23657864 Free PMC article.
-
The Cus efflux system removes toxic ions via a methionine shuttle.Protein Sci. 2011 Jan;20(1):6-18. doi: 10.1002/pro.532. Protein Sci. 2011. PMID: 20981744 Free PMC article. Review.
-
Chimeric analysis of the multicomponent multidrug efflux transporters from gram-negative bacteria.J Bacteriol. 2002 Dec;184(23):6499-507. doi: 10.1128/JB.184.23.6499-6507.2002. J Bacteriol. 2002. PMID: 12426337 Free PMC article.
-
Role of AcrAB-TolC and Its Components in Influx-Efflux Dynamics of QAC Drugs in Escherichia coli Revealed Using SHG Spectroscopy.J Phys Chem Lett. 2024 Aug 8;15(31):7832-7839. doi: 10.1021/acs.jpclett.4c01189. Epub 2024 Jul 25. J Phys Chem Lett. 2024. PMID: 39052610
Cited by
-
Does Chlorination Promote Antimicrobial Resistance in Waterborne Pathogens? Mechanistic Insight into Co-Resistance and Its Implication for Public Health.Antibiotics (Basel). 2022 Apr 22;11(5):564. doi: 10.3390/antibiotics11050564. Antibiotics (Basel). 2022. PMID: 35625208 Free PMC article. Review.
-
Potential therapeutic targets from Mycobacterium abscessus (Mab): recently reported efforts towards the discovery of novel antibacterial agents to treat Mab infections.RSC Med Chem. 2022 Mar 10;13(4):392-404. doi: 10.1039/d1md00359c. eCollection 2022 Apr 20. RSC Med Chem. 2022. PMID: 35647542 Free PMC article. Review.
-
Structural Insights into the Niemann-Pick C1 (NPC1)-Mediated Cholesterol Transfer and Ebola Infection.Cell. 2016 Jun 2;165(6):1467-1478. doi: 10.1016/j.cell.2016.05.022. Epub 2016 May 26. Cell. 2016. PMID: 27238017 Free PMC article.
-
Success stories of natural product-derived compounds from plants as multidrug resistance modulators in microorganisms.RSC Adv. 2023 Mar 8;13(12):7798-7817. doi: 10.1039/d3ra00184a. eCollection 2023 Mar 8. RSC Adv. 2023. PMID: 36909750 Free PMC article. Review.
-
Implication of the σE Regulon Members OmpO and σN in the ΔompA299-356-Mediated Decrease of Oxidative Stress Tolerance in Stenotrophomonas maltophilia.Microbiol Spectr. 2023 Aug 17;11(4):e0108023. doi: 10.1128/spectrum.01080-23. Epub 2023 Jun 7. Microbiol Spectr. 2023. PMID: 37284772 Free PMC article.
References
-
- Akama H, Kanemaki M, Yoshimura M, Tsukihara T, Kashiwagi T, et al. Crystal structure of the drug discharge outer membrane protein, OprM, of Pseudomonas aeruginosa: dual modes of membrane anchoring and occluded cavity end. J Biol Chem. 2004;279(51):52816–9. - PubMed
-
- Akama H, Matsuura T, Kashiwagi S, Yoneyama H, Narita S, et al. Crystal structure of the membrane fusion protein, MexA, of the multidrug transporter in Pseudomonas aeruginosa. J Biol Chem. 2004;279(25):25939–42. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

