Coactivator MYST1 regulates nuclear factor-κB and androgen receptor functions during proliferation of prostate cancer cells
- PMID: 24702180
- PMCID: PMC4042067
- DOI: 10.1210/me.2014-1055
Coactivator MYST1 regulates nuclear factor-κB and androgen receptor functions during proliferation of prostate cancer cells
Abstract
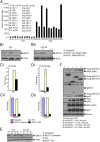
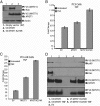
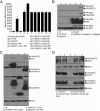
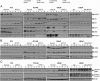
In prostate cancer (PCa), the functional synergy between androgen receptor (AR) and nuclear factor-κ B (NF-κB) escalates the resistance to therapeutic regimens and promotes aggressive tumor growth. Although the underlying mechanisms are less clear, gene regulatory abilities of coactivators can bridge the transcription functions of AR and NF-κB. The present study shows that MYST1 (MOZ, YBF2 and SAS2, and TIP60 protein 1) costimulates AR and NF-κB functions in PCa cells. We demonstrate that activation of NF-κB promotes deacetylation of MYST1 by sirtuin 1. Further, the mutually exclusive interactions of MYST1 with sirtuin 1 vs AR regulate the acetylation of lysine 16 on histone H4. Notably, in AR-lacking PC3 cells and in AR-depleted LNCaP cells, diminution of MYST1 activates the cleavage of poly(ADP-ribose) polymerase and caspase 3 that leads to apoptosis. In contrast, in AR-transformed PC3 cells (PC3-AR), depletion of MYST1 induces cyclin-dependent kinase (CDK) N1A/p21, which results in G2M arrest. Concomitantly, the levels of phospho-retinoblastoma, E2F1, CDK4, and CDK6 are reduced. Finally, the expression of tumor protein D52 (TPD52) was unequivocally affected in PC3, PC3-AR, and LNCaP cells. Taken together, the results of this study reveal that the functional interactions of MYST1 with AR and NF-κB are critical for PCa progression.
Figures



Similar articles
-
Lysine Acetyltransferases and Their Role in AR Signaling and Prostate Cancer.Front Endocrinol (Lausanne). 2022 Aug 17;13:886594. doi: 10.3389/fendo.2022.886594. eCollection 2022. Front Endocrinol (Lausanne). 2022. PMID: 36060957 Free PMC article. Review.
-
Interplay of nuclear factor-kappaB and B-myb in the negative regulation of androgen receptor expression by tumor necrosis factor alpha.Mol Endocrinol. 2008 Feb;22(2):273-86. doi: 10.1210/me.2007-0332. Epub 2007 Nov 1. Mol Endocrinol. 2008. PMID: 17975021 Free PMC article.
-
Tip60 promotes prostate cancer cell proliferation by translocation of androgen receptor into the nucleus.Prostate. 2010 Apr 1;70(5):540-54. doi: 10.1002/pros.21088. Prostate. 2010. PMID: 19938016
-
Identification of a negative regulatory cis-element in the enhancer core region of the prostate-specific antigen promoter: implications for intersection of androgen receptor and nuclear factor-kappaB signalling in prostate cancer cells.Biochem J. 2004 Apr 15;379(Pt 2):421-31. doi: 10.1042/BJ20031661. Biochem J. 2004. PMID: 14715080 Free PMC article.
-
Expression and function of androgen receptor coactivators in prostate cancer.J Steroid Biochem Mol Biol. 2004 Nov;92(4):265-71. doi: 10.1016/j.jsbmb.2004.10.003. Epub 2004 Dec 19. J Steroid Biochem Mol Biol. 2004. PMID: 15663989 Review.
Cited by
-
MOF upregulates the estrogen receptor α signaling pathway by its acetylase activity in hepatocellular carcinoma.Cancer Sci. 2021 May;112(5):1865-1877. doi: 10.1111/cas.14836. Epub 2021 Mar 30. Cancer Sci. 2021. PMID: 33544437 Free PMC article.
-
Lysine Acetyltransferase 8: A Target for Natural Compounds in Cancer Therapy.Int J Mol Sci. 2025 May 29;26(11):5257. doi: 10.3390/ijms26115257. Int J Mol Sci. 2025. PMID: 40508066 Free PMC article. Review.
-
Androgen Receptor-Interacting Proteins in Prostate Cancer Development and Therapy Resistance.Am J Pathol. 2024 Mar;194(3):324-334. doi: 10.1016/j.ajpath.2023.12.003. Epub 2023 Dec 15. Am J Pathol. 2024. PMID: 38104650 Free PMC article. Review.
-
Lysine Acetyltransferases and Their Role in AR Signaling and Prostate Cancer.Front Endocrinol (Lausanne). 2022 Aug 17;13:886594. doi: 10.3389/fendo.2022.886594. eCollection 2022. Front Endocrinol (Lausanne). 2022. PMID: 36060957 Free PMC article. Review.
-
The Versatility of Sirtuin-1 in Endocrinology and Immunology.Front Cell Dev Biol. 2020 Nov 19;8:589016. doi: 10.3389/fcell.2020.589016. eCollection 2020. Front Cell Dev Biol. 2020. PMID: 33330467 Free PMC article. Review.
References
-
- Shiota M, Yokomizo A, Masubuchi D, et al. Tip60 promotes prostate cancer cell proliferation by translocation of androgen receptor into the nucleus. Prostate. 2010;70:540–554. - PubMed
-
- Cronauer MV, Culig Z. Molecular aspects of prostate cancer. World J Urol. 2012;30:277–278. - PubMed
-
- Culig Z, Santer FR. Androgen receptor co-activators in the regulation of cellular events in prostate cancer. World J Urol. 2012;30:297–302. - PubMed
-
- Gaughan L, Logan IR, Cook S, Neal DE, Robson CN. Tip60 and histone deacetylase 1 regulate androgen receptor activity through changes to the acetylation status of the receptor. J Biol Chem. 2002;277:25904–25913. - PubMed
-
- Halkidou K, Gnanapragasam VJ, Mehta PB, et al. Expression of Tip60, an androgen receptor coactivator, and its role in prostate cancer development. Oncogene. 2003;22:2466–2477. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

