Endoplasmic reticulum stress and oxidative stress in cell fate decision and human disease
- PMID: 24702237
- PMCID: PMC4076992
- DOI: 10.1089/ars.2014.5851
Endoplasmic reticulum stress and oxidative stress in cell fate decision and human disease
Abstract
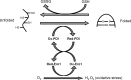
Significance: The endoplasmic reticulum (ER) is a specialized organelle for the folding and trafficking of proteins, which is highly sensitive to changes in intracellular homeostasis and extracellular stimuli. Alterations in the protein-folding environment cause accumulation of misfolded proteins in the ER that profoundly affect a variety of cellular signaling processes, including reduction-oxidation (redox) homeostasis, energy production, inflammation, differentiation, and apoptosis. The unfolded protein response (UPR) is a collection of adaptive signaling pathways that evolved to resolve protein misfolding and restore an efficient protein-folding environment.
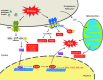
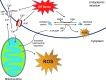
Recent advances: Production of reactive oxygen species (ROS) has been linked to ER stress and the UPR. ROS play a critical role in many cellular processes and can be produced in the cytosol and several organelles, including the ER and mitochondria. Studies suggest that altered redox homeostasis in the ER is sufficient to cause ER stress, which could, in turn, induce the production of ROS in the ER and mitochondria.
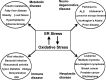
Critical issues: Although ER stress and oxidative stress coexist in many pathologic states, whether and how these stresses interact is unknown. It is also unclear how changes in the protein-folding environment in the ER cause oxidative stress. In addition, how ROS production and protein misfolding commit the cell to an apoptotic death and contribute to various degenerative diseases is unknown.
Future directions: A greater fundamental understanding of the mechanisms that preserve protein folding homeostasis and redox status will provide new information toward the development of novel therapeutics for many human diseases.
Figures

References
-
- Almenier HA, Al Menshawy HH, Maher MM, and Al Gamal S. Oxidative stress and inflammatory bowel disease. Front Biosci (Elite Ed) 4: 1335–1344, 2012 - PubMed
-
- Atkins C, Liu Q, Minthorn E, Zhang SY, Figueroa DJ, Moss K, Stanley TB, Sanders B, Goetz A, Gaul N, Choudhry AE, Alsaid H, Jucker BM, Axten JM, and Kumar R. Characterization of a novel PERK kinase inhibitor with antitumor and antiangiogenic activity. Cancer Res 73: 1993–2002, 2013 - PubMed
-
- This reference has been deleted.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

