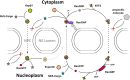
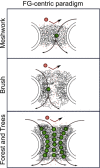
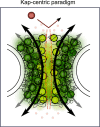
Disordered proteinaceous machines
- PMID: 24702702
- PMCID: PMC4350607
- DOI: 10.1021/cr4007329
Disordered proteinaceous machines
Erratum in
-
Correction to disordered proteinaceous machines.Chem Rev. 2015 Apr 8;115(7):2780. doi: 10.1021/acs.chemrev.5b00150. Epub 2015 Mar 26. Chem Rev. 2015. PMID: 25811425 Free PMC article. No abstract available.
Figures




References
-
- Wright P. E.; Dyson H. J. J. Mol. Biol. 1999, 293, 321. - PubMed
-
- Uversky V. N.; Gillespie J. R.; Fink A. L. Proteins 2000, 41, 415. - PubMed
-
- Dunker A. K.; Lawson J. D.; Brown C. J.; Williams R. M.; Romero P.; Oh J. S.; Oldfield C. J.; Campen A. M.; Ratliff C. M.; Hipps K. W.; Ausio J.; Nissen M. S.; Reeves R.; Kang C.; Kissinger C. R.; Bailey R. W.; Griswold M. D.; Chiu W.; Garner E. C.; Obradovic Z. J. Mol. Graphics Modell. 2001, 19, 26. - PubMed
-
- Dunker A. K.; Obradovic Z. Nat. Biotechnol. 2001, 19, 805. - PubMed
-
- Dyson H. J.; Wright P. E. Curr. Opin. Struct. Biol. 2002, 12, 54. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

