Involvement of H1 and H2 receptors and soluble guanylate cyclase in histamine-induced relaxation of rat mesenteric collecting lymphatics
- PMID: 24702851
- PMCID: PMC4185265
- DOI: 10.1111/micc.12138
Involvement of H1 and H2 receptors and soluble guanylate cyclase in histamine-induced relaxation of rat mesenteric collecting lymphatics
Abstract
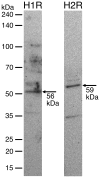
Objective: This study investigated the roles of the H1 and H2 histamine receptors, NO synthase, and sGC cyclase in histamine-induced modulation of rat mesenteric collecting lymphatic pumping.
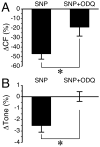
Methods: Isolated rat mesenteric collecting lymphatics were treated with 1- to 100-μM histamine. Histamine receptors were blocked with either the H1 antagonist mepyramine or the H2 antagonist cimetidine. The role of NO/sGC signaling was tested using the arginine analog L-NAME, the sGC inhibitor ODQ, and SNP as a positive control.
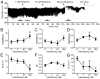
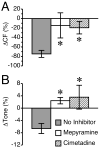
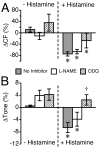
Results: Histamine applied at 100 μM decreased tone and CF of isolated rat mesenteric collecting lymphatics. Pharmacologic blockade of either H1 or H2 histamine receptors significantly inhibited the response to histamine. Pretreatment with ODQ, but not L-NAME, completely inhibited the histamine-induced decrease in tone. ODQ pretreatment also significantly inhibited SNP-induced lymphatic relaxation.
Conclusions: H1 and H2 histamine receptors are both involved in histamine-induced relaxation of rat mesenteric collecting lymphatics. NO synthesis does not appear to contribute to the histamine-induced response. However, sGC is critical for the histamine-induced decrease in tone and contributes to the drop in CF.
Keywords: endothelium; lymph flow; lymphatic pump; signal transduction.
© 2014 John Wiley & Sons Ltd.
Figures



Comment in
-
Itching for answers: how histamine relaxes lymphatic vessels.Microcirculation. 2014 Oct;21(7):575-7. doi: 10.1111/micc.12162. Microcirculation. 2014. PMID: 25123019 Free PMC article.
References
-
- Alitalo K. The lymphatic vasculature in disease. Nat Med. 2011;17:1371–1380. - PubMed
-
- Beermann S, Seifert R, Neumann D. Commercially available antibodies against human and murine histamine H(4)-receptor lack specificity. Naunyn Schmiedebergs Arch Pharmacol. 2012;385:125–135. - PubMed
-
- Chambers R, Zweifach BW. Intercellular cement and capillary permeability. Physiol Rev. 1947;27:436–463. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

