Role of the hippocampus in Nav1.6 (Scn8a) mediated seizure resistance
- PMID: 24704313
- PMCID: PMC4077284
- DOI: 10.1016/j.nbd.2014.03.014
Role of the hippocampus in Nav1.6 (Scn8a) mediated seizure resistance
Abstract
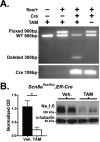
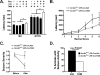
SCN1A mutations are the main cause of the epilepsy disorders Dravet syndrome (DS) and genetic epilepsy with febrile seizures plus (GEFS+). Mutations that reduce the activity of the mouse Scn8a gene, in contrast, are found to confer seizure resistance and extend the lifespan of mouse models of DS and GEFS+. To investigate the mechanism by which reduced Scn8a expression confers seizure resistance, we induced interictal-like burst discharges in hippocampal slices of heterozygous Scn8a null mice (Scn8a(med/+)) with elevated extracellular potassium. Scn8a(med/+) mutants exhibited reduced epileptiform burst discharge activity after P20, indicating an age-dependent increased threshold for induction of epileptiform discharges. Scn8a deficiency also reduced the occurrence of burst discharges in a GEFS+ mouse model (Scn1a(R1648H/+)). There was no detectable change in the expression levels of Scn1a (Nav1.1) or Scn2a (Nav1.2) in the hippocampus of adult Scn8a(med/+) mutants. To determine whether the increased seizure resistance associated with reduced Scn8a expression was due to alterations that occurred during development, we examined the effect of deleting Scn8a in adult mice. Global Cre-mediated deletion of a heterozygous floxed Scn8a allele in adult mice was found to increase thresholds to chemically and electrically induced seizures. Finally, knockdown of Scn8a gene expression in the adult hippocampus via lentiviral Cre injection resulted in a reduction in the number of EEG-confirmed seizures following the administration of picrotoxin. Our results identify the hippocampus as an important structure in the mediation of Scn8a-dependent seizure protection and suggest that selective targeting of Scn8a activity might be efficacious in patients with epilepsy.
Keywords: Cre recombinase; Dravet syndrome; GEFS+; Lentivirus; Na(v)1.6; Nav1.1; Scn1a; Scn8a; Voltage-gated sodium channel.
Copyright © 2014 Elsevier Inc. All rights reserved.
Figures




Similar articles
-
An Scn1a epilepsy mutation in Scn8a alters seizure susceptibility and behavior.Exp Neurol. 2016 Jan;275 Pt 1(0 1):46-58. doi: 10.1016/j.expneurol.2015.09.008. Epub 2015 Sep 26. Exp Neurol. 2016. PMID: 26410685 Free PMC article.
-
Neuronal voltage-gated ion channels are genetic modifiers of generalized epilepsy with febrile seizures plus.Neurobiol Dis. 2011 Mar;41(3):655-60. doi: 10.1016/j.nbd.2010.11.016. Epub 2010 Dec 13. Neurobiol Dis. 2011. PMID: 21156207 Free PMC article.
-
The voltage-gated sodium channel Scn8a is a genetic modifier of severe myoclonic epilepsy of infancy.Hum Mol Genet. 2007 Dec 1;16(23):2892-9. doi: 10.1093/hmg/ddm248. Epub 2007 Sep 19. Hum Mol Genet. 2007. PMID: 17881658
-
Sodium Channelopathies in Human and Animal Models of Epilepsy and Neurodevelopmental Disorders.In: Noebels JL, Avoli M, Rogawski MA, Vezzani A, Delgado-Escueta AV, editors. Jasper's Basic Mechanisms of the Epilepsies. 5th edition. New York: Oxford University Press; 2024. Chapter 44. In: Noebels JL, Avoli M, Rogawski MA, Vezzani A, Delgado-Escueta AV, editors. Jasper's Basic Mechanisms of the Epilepsies. 5th edition. New York: Oxford University Press; 2024. Chapter 44. PMID: 39637100 Free Books & Documents. Review.
-
Dravet syndrome and its mimics: Beyond SCN1A.Epilepsia. 2017 Nov;58(11):1807-1816. doi: 10.1111/epi.13889. Epub 2017 Sep 7. Epilepsia. 2017. PMID: 28880996 Review.
Cited by
-
The NaV1.7 Channel Subtype as an Antinociceptive Target for Spider Toxins in Adult Dorsal Root Ganglia Neurons.Front Pharmacol. 2018 Sep 4;9:1000. doi: 10.3389/fphar.2018.01000. eCollection 2018. Front Pharmacol. 2018. PMID: 30233376 Free PMC article. Review.
-
Distinctive Properties and Powerful Neuromodulation of Nav1.6 Sodium Channels Regulates Neuronal Excitability.Cells. 2021 Jun 25;10(7):1595. doi: 10.3390/cells10071595. Cells. 2021. PMID: 34202119 Free PMC article. Review.
-
Prominent role of forebrain excitatory neurons in SCN8A encephalopathy.Brain. 2019 Feb 1;142(2):362-375. doi: 10.1093/brain/awy324. Brain. 2019. PMID: 30601941 Free PMC article.
-
Differential roles of NaV1.2 and NaV1.6 in regulating neuronal excitability at febrile temperature and distinct contributions to febrile seizures.Sci Rep. 2018 Jan 15;8(1):753. doi: 10.1038/s41598-017-17344-8. Sci Rep. 2018. PMID: 29335582 Free PMC article.
-
Pro-excitatory alterations in sodium channel activity facilitate subiculum neuron hyperexcitability in temporal lobe epilepsy.Neurobiol Dis. 2017 Dec;108:183-194. doi: 10.1016/j.nbd.2017.08.018. Epub 2017 Aug 30. Neurobiol Dis. 2017. PMID: 28860087 Free PMC article.
References
-
- Barton ME, Klein BD, Wolf HH, White HS. Pharmacological characterization of the 6 Hz psychomotor seizure model of partial epilepsy. Epilepsy research. 2001;47:217–227. - PubMed
-
- Brown CW. Properties and Alterations of Electrically-Induced Seizures in Mice. Epilepsia. 1953;C2:127–138.
-
- Burgess DL, Kohrman DC, Galt J, Plummer NW, Jones JM, Spear B, Meisler MH. Mutation of a new sodium channel gene, Scn8a, in the mouse mutant ‘motor endplate disease’. Nat Genet. 1995;10:461–465. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
