Cholesterol in the retina: the best is yet to come
- PMID: 24704580
- PMCID: PMC4058366
- DOI: 10.1016/j.preteyeres.2014.03.002
Cholesterol in the retina: the best is yet to come
Abstract
Historically understudied, cholesterol in the retina is receiving more attention now because of genetic studies showing that several cholesterol-related genes are risk factors for age-related macular degeneration (AMD) and because of eye pathology studies showing high cholesterol content of drusen, aging Bruch's membrane, and newly found subretinal lesions. The challenge before us is determining how the cholesterol-AMD link is realized. Meeting this challenge will require an excellent understanding these genes' roles in retinal physiology and how chorioretinal cholesterol is maintained. In the first half of this review, we will succinctly summarize physico-chemical properties of cholesterol, its distribution in the human body, general principles of maintenance and metabolism, and differences in cholesterol handling in human and mouse that impact on experimental approaches. This information will provide a backdrop to the second part of the review focusing on unique aspects of chorioretinal cholesterol homeostasis, aging in Bruch's membrane, cholesterol in AMD lesions, a model for lesion biogenesis, a model for macular vulnerability based on vascular biology, and alignment of AMD-related genes and pathobiology using cholesterol and an atherosclerosis-like progression as unifying features. We conclude with recommendations for the most important research steps we can take towards delineating the cholesterol-AMD link.
Keywords: Age-related macular degeneration; Cholesterol; Choroid; Drusen; Lipoproteins; Retina.
Copyright © 2014 Elsevier Ltd. All rights reserved.
Figures
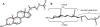









References
-
- Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report. Circulation. 2002;106:3143–3421. - PubMed
-
- Lutein + zeaxanthin and omega-3 fatty acids for age-related macular degeneration: the Age-Related Eye Disease Study 2 (AREDS2) randomized clinical trial. J. Amer. Med. Assoc. 2013;309:2005–2015. - PubMed
-
- Acton JH, et al. Drusen detection in retro-mode imaging by a scanning laser ophthalmoscope. Acta Ophthalmol. 2011;89:e404–411. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

