ICAM-2 confers a non-metastatic phenotype in neuroblastoma cells by interaction with α-actinin
- PMID: 24704826
- PMCID: PMC6746311
- DOI: 10.1038/onc.2014.87
ICAM-2 confers a non-metastatic phenotype in neuroblastoma cells by interaction with α-actinin
Abstract
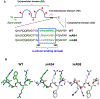
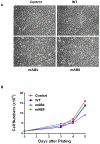
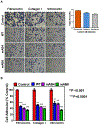
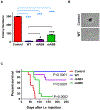
Progressive metastatic disease is a major cause of mortality for patients diagnosed with multiple types of solid tumors. One of the long-term goals of our laboratory is to identify molecular interactions that regulate metastasis, as a basis for developing agents that inhibit this process. Toward this goal, we recently demonstrated that intercellular adhesion molecule-2 (ICAM-2) converted neuroblastoma (NB) cells from a metastatic to a non-metastatic phenotype, a previously unknown function for ICAM-2. Interestingly, ICAM-2 suppressed metastatic but not tumorigenic potential in preclinical models, supporting a novel mechanism of regulating metastasis. We hypothesized that the effects of ICAM-2 on NB cell phenotype depend on the interaction of ICAM-2 with the cytoskeletal linker protein α-actinin. The goal of the study presented here was to evaluate the impact of α-actinin binding to ICAM-2 on the phenotype of NB tumor cells. We used in silico approaches to examine the likelihood that the cytoplasmic domain of ICAM-2 binds directly to α-actinin. We then expressed variants of ICAM-2 with mutated α-actinin-binding domains, and compared the impact of ICAM-2 and each variant on NB cell adhesion, migration, anchorage-independent growth, co-precipitation with α-actinin and production of localized and disseminated tumors in vivo. The in vitro and in vivo characteristics of cells expressing ICAM-2 variants with modified α-actinin-binding domains differed from cells expressing ICAM-2 wild type (WT) and also from cells that expressed no detectable ICAM-2. Like the WT protein, ICAM-2 variants inhibited cell adhesion, migration and colony growth in vitro. However, unlike the WT protein, ICAM-2 variants did not completely suppress development of disseminated NB tumors in vivo. The data suggest the presence of α-actinin-dependent and α-actinin-independent mechanisms, and indicate that the interaction of ICAM-2 with α-actinin is critical to conferring an ICAM-2-mediated non-metastatic phenotype in NB cells.
Conflict of interest statement
Conflict of interest.
The authors declare no conflict of interest.
Figures




References
-
- Casasnovas JM, Springer TA, Liu JH, Harrison SC, Wang JH. Crystal structure of ICAM-2 reveals a distinctive integrin recognition surface. Nature 1997; 387: 312–315. - PubMed
-
- Nortamo P, Li R, Renkonen R, Timonen T, Prieto J, Patarroyo M et al. The expression of human intercellular adhesion molecule-2 is refractory to inflammatory cytokines. Eur J Immunol 1991; 21: 2629–2632. - PubMed
-
- Kotovuori A, Pessa-Morikawa T, Kotovuori P, Nortamo P, Gahmberg CG. ICAM-2 and a peptide from its binding domain are efficient activators of leukocyte adhesion and integrin affinity. J Immunol 1999; 162: 6613–6620. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

