Developmental immunolocalization of the Klotho protein in mouse kidney epithelial cells
- PMID: 24704992
- PMCID: PMC3980205
- DOI: 10.4081/ejh.2014.2256
Developmental immunolocalization of the Klotho protein in mouse kidney epithelial cells
Abstract
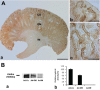
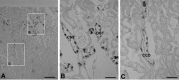
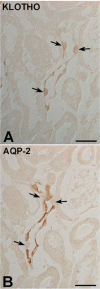
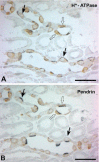
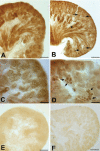
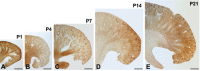
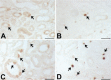
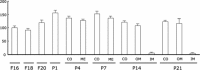
A defect in Klotho gene expression in the mouse results in a syndrome that resembles rapid human aging. In this study, we investigated the detailed distribution and the time of the first appearance of Klotho in developing and adult mouse kidney. Kidneys from 16-(F16), 18-(F18) and 20-day-old (F20) fetuses, 1- (P1), 4- (P4), 7- (P7), 14- (P14), and 21-day-old (P21) pups and adults were processed for immunohistochemistry and immunoblot analyses. In the developing mouse kidney, Klotho immunoreactivity was initially observed in a few cells of the connecting tubules (CNT) of 18-day-old fetus (F) and in the medullary collecting duct (MCD) and distal nephron of the F16 developing kidney. In F20, Klotho immunoreactivity was increased in CNT and additionally observed in the outer portion of MCD and tip of the renal papilla. During the first 3 weeks after birth, Klotho-positive cells gradually disappeared from the MCD due to apoptosis, but remained in the CNT and cortical collecting ducts (CCD). In the adult mouse, the Klotho protein was expressed only in a few cells of the CNT and CCD in cortical area. Also, Klotho immunoreactivity was observed in the aquaporin 2-positive CNT, CCD, and NaCl co-transporter-positive distal convoluted tubule (DCT) cells and type B and nonA-nonB intercalated cells of CNT, DCT, and CCD. Collectively, our data indicate that immunolocalization of Klotho is closely correlated with proliferation in the intercalated cells of CNT and CCD from aging, and may be involved in the regulation of tubular proliferation.
Conflict of interest statement
Conflict of interest: the authors declare no conflict of interests.
Figures

References
-
- Kuro-o M, Matsumura Y, Aizawa H, Kawaguchi H, Suga T, Utsugi T, et al. Mutation of the mouse klotho gene leads to a syndrome resembling ageing. Nature 1997;390:45-51 - PubMed
-
- Yoshida T, Fujimori T, Nabeshima Y. Mediation of unusually high concentrations of 1,25-dihydroxyvitamin D in homozygous klotho mutant mice by increased expression of renal 1alpha-hydroxylase gene. Endocrinology 2002; 143:683-9 - PubMed
-
- Mitani H, Ishizaka N, Aizawa T, Ohno M, Usui S, Suzuki T, et al. In vivo klotho gene transfer ameliorates angiotensin II-induced renal damage. Hypertension 2002; 39:838-43 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

